
Fig.1.3.1. Basic scheme of a nuclear reaction caused by a particle bombarding a nucleus.
| AstroNuclPhysics ® Nuclear Physics - Astrophysics - Cosmology - Philosophy | Physics and nuclear medicine |
1.
Nuclear and radiation physics
1.0. Physics - fundamental natural
science
1.1. Atoms and atomic nuclei
1.2. Radioactivity
1.3. Nuclear reactions and nuclear energy
1.4. Radionuclides
1.5. Elementary particle and accelerators
1.6. Ionizing radiation
1.3. Nuclear reactions and nuclear energy
Spontaneous decay or
transformation of nuclei, ie radioactivity, is
only one of the nuclear processes leading to the transmutation
of nuclei and the emission of ionizing radiation. Here
we will briefly discuss other nuclear processes associated with
the transformation of nuclei - nuclear reaction,
including the possibility of obtaining energy
from atomic nuclei.
Terminological
note:
The nuclear reactions discussed in this chapter are, in a sense,
a special case of "reactions" or interactions
of microworld particles. At least in the sense
that they are due to the interactions of the elementary
particles - protons, neutrons, electrons, photons,
possibly also mesons and hyperons. The category of nuclear
reactions also sometimes includes spontaneous decay or
transformation of nuclei, ie radioactivity, or the
interaction of protons and neutrons with each other or with other
particles. In our explanation, we have devoted a separate §1.2
"Radioactivity" to radioactivity and we deal with the interaction of particles in §1.5
"Elementary particles". In the text of this chapter we will deal with
nuclear reactions in the true sense of the word, ie
processes in atomic nuclei caused by interactions with other
particles or nuclei, mostly reactions in binary collisions
of nuclei and particles, including fission of heavy
atomic nuclei and fusion light cores to heavier cores.
In the school literature, nuclear
reactions are sometimes divided, according to the number of
nuclei and reacting particles involved, into two groups :
- Mononuclear
reactions involving a single atomic nucleus that
transforms to form a new nucleus and emitted particles
(corpuscular alpha, beta, or photons gamma). This includes
radioactivity.
- Binuclear reactions,
where the target nucleus, after interacting with a bombardment
particle or another nucleus, transforms into a new nucleus and
emitted particles (corpuscular or gamma photons).
We do not use this division and terminology here.
Chemical <- versus -> nuclear reactions
In chemical reactions, the electron shells of atoms
interact (§1.1, passage "Interactions of atoms") , in which atoms can
combine into molecules or, conversely, molecules can decompose.
In nuclear reactions, atomic nuclei interact with other
nuclei or particles to form new nuclei and emitted particles. The
course of specific types of nuclear reactions will be discussed
below. Analogies and differences between chemical and nuclear
reactions will also be discussed in several places.
Under nuclear reactions in nuclear physics generally refers to the process whereby two nucleons or two cores or nucleon or other particles and core approach each other at a distance of the order of 10-13 cm, enters the field of action of strong interactions, causing the nuclei changes in the number, energies and configurations of nucleons that may lead to the emission of other particles. The result is the transmutation of the nucleus - either to another isotope of the same element (change in the number of neutrons), or to the nucleus of another element (change in the number of protons). A new nucleus is almost always formed in the excited state, during its deexcitation g radiation is emitted. Cores transformed with nuclear reactions are often radioactive (mostly b- or b+); nuclear reactions are therefore the most important way of producing artificial radionuclides (see §1.4 "Radionuclides", section "Production of artificial radionuclides").

Fig.1.3.1. Basic scheme of a nuclear reaction caused by a
particle bombarding a nucleus.
Most nuclear
reactions involve the target nucleus being bombarded
by a particle. which by its interaction causes the change in the
nucleus and emission of new particles; such a reaction can be
written by a simple scheme *)
a + X ® Y
+ b + Q ,
where a denotes the arriving particle, X
the target nucleus, Y the nucleus
formed in the reaction, b the emitted particle
(it can also be a photon, or there can be emitted even several
particles), Q expresses the energy balance, ie the energy
released in an exothermic reaction or the energy delivered in an
endothermic reaction. This scheme is abbreviated as X(a,b)Y,
or even just (a,b), as far as
the reaction itself is concerned and not its products.
*) Slightly different schemes govern the reactions of nuclear
fission and nuclear
fusion, which will be
discussed in detail below in separate sections of this chapter.
The nuclei and particles involved
are provided with the indices N - number of nucleons and Z
- number of protons in the equations of nuclear reactions. Due to
the conservation laws (see below), the condition for the formal correctness of the
reaction equation is that the sum of the proton and neutron
numbers of the individual nuclei and particles on both sides of
the equation is the same.
Nuclear reactions are very diverse.
The same input situation - bombarding the same nuclei with the
same particles, often leads to different exit situations -
different interactions, in which different nuclei and various
particles are formed, with different probabilities.
Technical and
natural-scientific importance of nuclear reactions
Transmutation of elements - nuclear "alchemy"
A remarkable feature of nuclear reactions is that, in principle,
they make it possible to realize the ancient dream of alchemists
- the transmutation of elements *). E.g. even
that magic gold 197Au79 can be created from mercury 198Hg80 (content 10% in natural mercury) by bombarding high-energy gamma radiation with photons in
the photonuclear reaction - either directly in the
reaction 198Hg80(g, p)197Au79, or in reaction 198Hg80(g,n)197Hg80, followed by radioactive transformation of the
mercury-197 nucleus by electron capture 197Hg80+e-(EC)®197Au79 (T1/2=2.7 days) into the
resulting stable isotope gold-197. From the isotope of mercury 196Hg80 (which, however, is contained in natural
mercury only in 0.15%), gold can be
prepared by neutron fusion 196Hg80(n,g)197Hg80, again with subsequent radioactive transformation by
electron capture into the resulting stable isotope of gold.
Similarly, other elements can be created by nuclear reactions
from neighboring elements of the Mendeleean table. More
laboriously, a series of successive neutron fusions with
subsequent b- decays can create a number of heavier elements from
lighter elements, similar to the supernova explosion (§4.2 "Final stages of stellar
evolution. Gravitational collapse" of the book "Gravitation, black holes
and physics of space-time" and the syllabus "Cosmic Alchemy") .
*) However, alchemists had no idea not only
about atoms and their nuclei, but also did not recognize elements
and compounds. They judged the substances according to their
external manifestations and a few simple chemical reactions that
they were able to carry out (cf. the passage "Quackery
versus science" in the
above-mentioned monograph).
A common disadvantage of artificial
transmutation is the low yield, many orders of
magnitude lower than in the formation of compounds by chemical
reactions. In addition, the target material, or the final
product, usually needs to be purified by complex
methods, including mass spectrometry - isotope separation. In
practice, therefore, only a very small amount of
the resulting element can be prepared on accelerators,
only on the order of picograms. Even for the rarest elements
(gold, platinum), their production by nuclear transmutations
would be many millions of times more expensive than
their extraction from natural sources (where
a large number of them were left by nuclear reactions during a
supernova explosion) . A somewhat more
favorable situation in the yield is in nuclear reactors,
where a massive neutron flux can produce, for example, light transurans
(such as plutonium) also in kilogram quantities (see "Nuclear
reactors", "Transurans" below); uranium fission also produces a large
number of medium-heavy elements, mostly in the form of b- -radioactive isotopes.
Preparation of artificial
radionuclides
The creation of elements by nuclear transmutations is important
in nuclear research, for elements (or their isotopes) that do not
occur in terrestrial nature, or in the preparation of special
targets for accelerators. Nuclear transmutations are most often
used for the production of artificial radionuclides
(§1.4., Section "Production
of artificial radionuclides"), used in many areas of science, technology, medicine.
Newly formed atoms just after the nuclear
reaction of the original nucleus are sometimes called nascent
atoms (lat. nascendi = birth). They have an initially deformed and excited
electron shell, they have a non-zero electric charge - they are
in the state of a positive or negative ion, they
have a high kinetic energy due to the
transmitted kinetic energy during the interaction ("hot
atoms"). It leads to high chemical reactivity of
nascent atoms after a nuclear reaction.
Nuclear energy
In nuclear reactions, part of the binding energy of
nucleons in atomic nuclei may be released and converted
into the kinetic-thermal energy of the substance. This occurs in
two processes :
1. Combining
(fusion) of light nuclei into heavier ones - eg hydrogen
nuclei into helium. These thermonuclear reactions are a source of
radiant energy of stars - it is described in §4.1, section
"Thermonuclear
reactions inside stars" of the monograph "Gravity, black holes and
space-time physics". Efforts to carry out thermonuclear
reactions for the technical recovery of nuclear energy are
described below in the section "Fusion
of atomic nuclei. Thermonuclear reactions.".
2. Fission of
heavy nuclei into lighter ones - eg uranium nuclei. This
technology is used in current nuclear power plants, it is
discussed below in the section "Fission of atomic nuclei".
Cosmic nucleosynthesis
The enormous natural-scientific significance of
nuclear reactions lies in the nuclear nucleosynthesis
of all elements (heavier than hydrogen) in
space. This cosmic nucleosynthesis took place in two phases :
- Primordial cosmological nucleosynthesis of light
elements at the beginning of the universe - in addition
to light hydrogen, it was deuterium, temporarily tritium,
especially helium and smaller amounts of
lithium, beryllium, boron. It is analyzed in more detail in
§5.4, section "Lepton era. Initial nucleosynthesis"
monograph "Gravity, black holes and space-time
physics".
- Thermonuclear synthesis of heavier elements in stars
- §4.1, part "Thermonuclear reactions inside stars"
in the same book.
For the creation of
elements in the universe, see also §1.1, passage "Cosmic alchemy"
and syllabus "Cosmic Alchemy". Thanks nuclear reactions are in the
universe heavier elements, that make produce a diverse structure
and later life.
Conservation
laws and energy balance of nuclear reactions
An important common aspect of nuclear reactions
are conservation laws - it is mainly the law of
conservation of electric charge, number of nucleons, energy (kinetic energy and rest energy in connection with
Einstein's relation E = mc2 equivalence of mass and energy),
then momentum, angular momentum, or parity and isospin. The fact
that these laws must be complied with in nuclear reactions has
some basic consequences, such as the ways ("channels")
in which a given reaction can take place and which it cannot.
Their energy balance
is of great importance for the implementation, course and use of
nuclear reactions. Essencialy important is the balance of the kinetic
energy of nuclear reaction Q = [Ek(Y)+Ek(b)] - [Ek(X)+Ek(a)], which is the difference of the total kinetic
energy Ek
of particles after the reaction and before the reaction (here there are only two components, generally it would
be the sum over all incoming and outgoing particles). It is thus the kinetic energy released
or consumed in the reaction. According to the
law of conservation of energy and Einstein's relation of
equivalence of mass and energy, this energy of a nuclear reaction
is also given by the difference of the sums of rest
masses of all particles before the reaction and after
the reaction Q = {[m0(X)+m0(a)] - [m0(Y)+m0(b)]}.c2. For atomic nuclei, this is the difference in the
so-called mass defect given by the binding energy of the
nucleus.
According to the energy of a nuclear
reaction, these reactions are divided into two groups :
¨ Endothermic
(endoenergetic) reactions Q < 0 ,
where the kinetic energy of interacting nuclei and particles is
"consumed" to change the internal state of nuclei or to
release or produce new particles.
¨ Exothermic
(exoenergic) reactions Q > 0 ,
where there is a "release" and gain of kinetic energy,
which is drawn from the binding energy of
nuclei.
Most nuclear interactions have
endoenergetic character. Important exoenergetic
interactions between light nuclear fusion and heavy nuclear
fission will be discussed below in the "Nuclear Energy" section. In order to carry out most nuclear
reactions, it is necessary for the incident particles to have a
relatively high kinetic energy of the order of a few MeV. This
energy is needed both to overcome the Coulomb electrostatic
repulsion (if the particle is positively charged; this does not
apply to neutrons) and to introduce the energy needed for the
relevant changes in the nuclear structure. Therefore, most
nuclear reactions are performed with particles accelerated to
high energies on accelerators - see §1.5,
section "Charged particle
accelerators".
General
mechanisms of particle interactions with atomic nuclei
If a flying particle (situation according to Fig.3.1.1 bottom
left) comes close to the atomic nucleus, there can be several
ways of its interaction with the nucleus,
depending on the type of particle and nucleus (including their
charge), the kinetic energy of the particle, on the impact factor
:
Mechanisms
of nuclear reactions
Nuclear reactions are usually very complex processes
in which "come into play" a number of factors of
properties of incident particles (especially their electric
charge and other reported interactions - strong, weak), their
energy, momentum - impact factor, as well as structure of
bombarded atomic nuclei. If the shelling particle penetrates the
area of the target nucleus, the interaction can take place in
basically two ways (at least according to our model ideas) :
Effective cross-section of nuclear
reactions
As with chemical reactions, nuclear reactions take place
differently "willingly" - with different efficiencies
or probabilities, depending on the type of reaction and the
energy of the particles. The probability of nuclear reactions can
be clearly expressed in a geometric way using the so-called effective
cross section of the reaction. The effective cross
section expresses the probability that the
bombardment particle will interact with the target core in a
given specific way.
The concept of the effective
cross-section is based on the illustrative idea that the target
core behaves as an "absorbing body" with a radius r
with respect to the incident particle, which the particle either
hits and the required reaction occurs, or does not hit them
(passes them, flies around) and the reaction does not occur -
Fig.1.3.2. The larger the radius r of this body, resp. its
effective area s = p .r2 - effective cross section, the greater
the probability of interaction (probability that the particle
"hits").
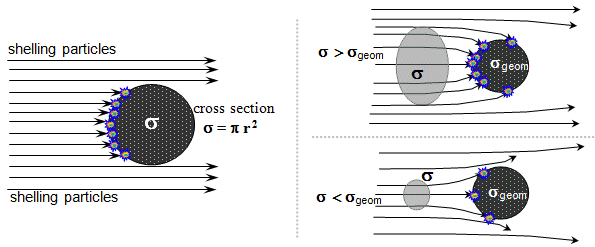
Fig.1.3.2 Expression of the probability of a nuclear reaction
using an effective cross section
The cross
section may, but need not be directly related to the
"geometric radius" of the target nucleus rgeom or its
"geometrical cross section" sgeom = p .r2geom. For "attracting" particles (eg neutrons) s > sgeom, for repelling particles (eg protons) is s < sgeom - Fig.1.3.2 on the right. In addition, the same firing
particle can cause different nuclear reactions on
the same core , the various probabilities of which are described by
different effective cross sections. These effective
cross-sections no longer have anything to do with the geometric
dimensions of the core - they are the result of the internal
mechanisms of specific types of reactions.
The so-called impact
parameter b is important for the course of a
specific interaction of nuclei: it is the geometric distance
of the centers of effective "disks" of interacting
particles (nuclei and particles), in which they fly around each
other or they intersect. In the case of a small impact parameter
b << rgeom it is a central collision, in the case
of larger values b it is a peripheral
collision. If the impact parameter is greater than rgeom , resp. greater
than the sum of the effective radii of booth nuclei, there are no
longer strong interactions between nucleons, but nuclei can
interact through their electric fields (such a collision is
sometimes called ultraperipheral).
The unit of effective cross-section
in the SI system would be m2, which is, however, inadequately large, and therefore
in practice the unit barn (bn) is used: 1 bn =
10-28 m2, which has the order
of magnitude of the geometric cross-section of heavy atomic
nuclei such as uranium nuclei.
Note: The slang
name "barn" originated in the
early nuclear technology in the 1940s from the humorous
comparison that neutrons hits "nuclei as big as a barn"
- uranium 235 nuclei.
The effective
cross section s indicates the probability that the bombardment particle
will interact with the target core in a give specific way. If we
were bombard a target substance, that has the number of SN of
the given atomic nuclei per unit area, by the total number no of particles that can
enter nuclear reactions, then the number of particles n
which actually cause a given nuclear reaction will be: n = no .SN .s. This can be
rewritten as s = (n/no). (1/SN) - cross section is given by the ratio of the realized
nuclear reactions to the number of particles needed to induce
them, multiplied by the inverse of the number of nuclei per unit
area substance.
Energy
dependence of nuclear reactions
The actual course and efficiency (effective cross-section) of
nuclear reactions depends in a complex way not only on the type
of target nucleus and the bombardment particle, but also on the
kinetic energy of this particle, more precisely on the
energy in the center of gravity system [nucleus +
particles]. With the exception of neutron capture, there is an energy
threshold for most nuclear reactions *); below this
value the reaction does not occur (or it
can occur with low probability, due to the quantum tunneling
phenomenon). With increasing energy,
different types of reactions then occur first with an increasing
effective cross-section, but then the effective cross-section
often decreases and one type of reaction is replaced by other
types. By a suitable setting of the energy of
the bombarded particles, the optimal effective cross-section can
be achieved for the specific nuclear reaction required. However,
even with the same energy, different types of reactions often
occur (albeit with different effective cross sections).
*) For positively charged particles
(protons, deuterons, a) the energy threshold is given mainly by the need to
overcome the repulsive electric (Coulomb) forces of a positively
charged nucleus. For photons, the energy threshold of
photonuclear reactions is given by the binding energy of nucleons
in specific nuclei.
Types of
nuclear reactions
Nuclear reactions are usually classified
according to the cause of their origin, ie what particles
they were caused by :
Neutron-induced
reactions
The easiest way to induce nuclear reactions is to use neutrons
that do not have an electric charge, are not repelled by nuclei,
and therefore usually willingly enter nuclei
even when they are slow *). The simplest neutron reaction is a
ordinary capture of a neutron by the nucleus X
- neutron fusion, which already remains in the nucleus: 1n0 + NXZ ® N+1YZ + g, while the newly formed composite nucleus Y
is in an excited state and deexcited by photon radiation g. Therefore, this
reaction is also called neutron radiation capture, and
X(n,g)Y,
or just (n,g), is abbreviated. The newly formed nucleus Y
is an isotope of the same element, enriched in one neutron; often
shows b- radioactivity.
*) The slower the neutrons fly on, the more
likely they are to penetrate the nucleus (they have "more
time" to do so). The effective neutron capture cross section
is therefore largest for very slow "thermal" neutrons.
With increasing kinetic energy of neutrons En (ie velocity of neutrons nn), the effective cross section first decreases
monotonically (due to the shorter residence
time of the neutron in the nucleus),
approximately according to the law 1/vn. In the region of slow neutrons around about tens of
keV, the energy dependence of the effective cross section shows a
number of sharp resonant maxima and minima (related to the occupancy of discrete energy levels of
nucleons in the nucleus; for heavy nuclei resonant maxima and
minima are more pronounced and very condensed), after which, for higher neutron energies, the
effective neutron capture cross section decreases significantly.
A typical energy dependence of the effective cross section of
neutron capture by the nucleus is in the left part of the figure
below.
Neutrons can
also induce other reactions in the nucleus associated with
particle radiation, especially at higher kinetic energies. Such
reactions are (n, p), (n, d), (n, a ), resp. at higher
energies, more particles can be emitted, such as (n, 2p), etc. Production
of radionuclides by neutron reactions is mentioned in
§1.4 "Radionuclides", part "Production of artificial
radionuclides". Nuclear
reactions induced by neutrons are further used in neutron
activation analysis (§3.4,
section "Activation
analysis").
For heavy uranium and transuranic
nuclei, neutrons induce specific fission
reactions, which will be discussed in detail below in the "Nuclear Fission" section.
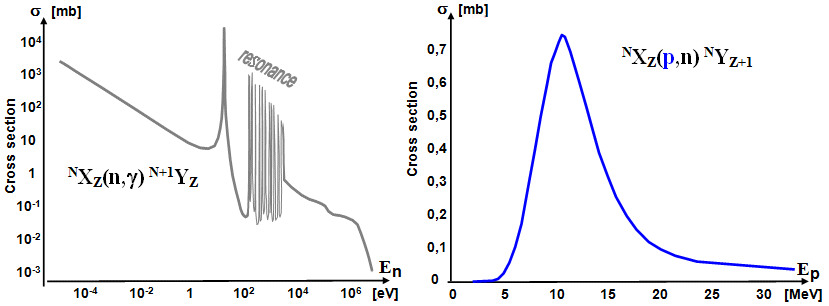
Typical dependence of the effective cross section of nuclear
reactions on the energy of bombarding neutrons (left) and protons
(right).
Nuclear reactions induced by
neutrons and protons are of great importance in nuclear
astrophysics - in primordial cosmological
nucleosynthesis (§5.4, part
"Lepton era. Initial nucleosynthesis" monograph "Gravity,
black holes and space-time physics")
and nucleosynthesis inside stars,
nova explosions and especially supernovae (it is discussed in §4.1, part "Evolution of stars" and in §4.2, part " Supernova explosion. Neutron
star. Pulsars.", passage
"Types of supernovae
and their astronomical classification" in the same book).
Reactions induced by protons
In order for the proton p+ penetrated into the nucleus and could cause a nuclear
reaction there, it must be accelerated *) to
relatively high kinetic energy (at least hundreds of keV to MeV
units) to overcome the repulsive electrical (Coulomb) forces of a
positively charged nucleus. Depending on the energy of the
protons, a number of reactions can take place. The simplest of
these is the radiation capture of a proton (p, g): p+ + NXZ ® N+1YZ+1 + g, but also occur reactions of the type (p, p), (p, n),
(p, d), (p, a), at higher energies more particles can be emitted, eg
(p, 2n), (p, pn), (p, 3n). The resulting Y core
often exhibits b+
-radioactivity (the nucleus is usually enriched in proton); the
production of radionuclides by proton reactions is mentioned in
§1.4 "Radionuclides", part "Production of artificial
radionuclides"
*) Acceleration of protons and other
charged particles (heavier ions) is most often performed in a cyclotron,
or in a linear accelerator - it is discussed in more detail in
§1.5 "Elementary particles", part "Charged particle accelerators").
To carry out a nuclear reaction, a
proton must have a certain threshold energy to
overcome the repulsive electrical force of the core, in
co-production with the tunneling phenomenon. Thus, with proton
energy, the effective cross-section of the reaction first
increases sharply from zero to a certain maximum value, and then
decreases monotonically again at higher energies, as
high-velocity protons shorten their residence time inside the
nucleus (reducing the likelihood of a
nuclear reaction).
At the highest proton energies
(hundreds of MeVs and more), fragmentation reactions
occur, in which the nucleus is more or less
"broken" - a larger number of protons and neutrons of
different energies are ejected from it; or other particles are
produced, most often p- mesons. In addition to accelerators, we encounter these
effects during the impact of cosmic radiation (§1.6 "Ionizing radiation", part "Cosmic radiation"); the interesting use of
the fragmentation reaction for so-called accelerator -
controlled transmutation technology (ADTT) is mentioned below.
Reactions
induced by deuterons, a-particles, heavier
nuclei (positive ions) :
- Deuterons
Anther relatively heavy particles can cause nuclear reactions,
are the ions-nuclei of deuterium 2H1, or deuterons d
formed by a pair of coupled protons and neutrons. The
most common reactions of deuterons with target nuclei are (d, p)
and (d, n), which take place mainly by direct processes of
"entrainment" of nucleons. Such direct processes take
place by tearing off and absorbing a neutron or
proton from the deuteron in the field of the atomic nucleus. This
is due to the relatively large distance » 4.10-13
cm between a proton and a neutron in
a deuteron and their lower binding force (corresponding to a
binding energy of 2.226 MeV). By reaction with
cyclotron-accelerated deuterons, it is often used to prepare
radionuclides, rarely as neutron sources.
Deuterium-tritium neutron generators
It is enough to accelerate the deuterons to an energy of about
100-200keV and let them fall on a target containing tritium to
cause a nuclear reaction 2D1
+ 3T1 ® 1n0
+ 4He2 (+17,6MeV), in which neutrons
are released. A fairly small accelerator or just a tube is enough
for this (see §1.5, section
"Accelerators", pasage "Accelerators as neutron generators"). Such neutron
generators are used in a number of applications,
especially in neutron activation analysis (§3.4, section "Neutron
activation analysis"), in some radiation technologies, experimentally also in
radiotherapy (§3.6,
section "Hadron radiotherapy").
- Alpha particles
Particles a, which are the nuclei of helium 4He2 induce during
bombardment of target nuclei most reactions of type (a, n) and (a, p), with event.
emissions of quantum g; both of these types of reactions occur with roughly
the same probability. In light nuclei, these reactions can also
take place with the energies of particles a the order of MeV units, which
occur in some natural radionuclides from the uranium and thorium
decay series. With reactions of type (a, p) already in 1919
E.Rutheford carried out the first artificial transformation of
elements, reactions (a, n) led to the discovery of the neutron by J.Chadwick
in 1932 during the bombardment of beryllium nuclei by alpha
particles. Alpha particles from the radionuclides of reactions (a, n) are still used
as neutron sources. Otherwise, however,
particles a artificially accelerated in accelerators, are now used
for nuclear reactions and the production of radionuclide, where
nuclear reactions can be performed for all elements of
Mendeleev's periodic table.
- Nuclear reactions in
collisions of heavier nuclei
Heavier nuclei, also referred to as multiple-charged ions
(eg lithium 7Li3 , ..., carbon 12C6,
nitrogen 14N7, oxygen 16O8, ..., neon 20N10, and others), due to
the high Coulomb repulsive barrier, it is necessary to accelerate
to considerably high kinetic energies in order to carry out a
nuclear reaction (> »100 MeV, the heavier
the core, the higher). At lower energies,
only electromagnetic (Coulomb) excitation of the nucleus occurs,
usually with a higher angular momentum. At energies only slightly
above the threshold energy, there is usually a peripheral
direct interaction of the ion with the nucleus, in which one
nucleon (neutron or proton) is transferred ("torn
down") from the ion to the nucleus. At higher energies, an
excited composite nucleus is formed with
subsequent "evaporative" emission of particles
(nucleons, a-particles). Multiple charged ions with sufficiently
high energy can further cause the heavier nuclei to split
into two lighter nuclei, event. fragmentation and breakage,
mostly with neutron emission and quantum g-radiation. Alternatively,
bombarding heavy nuclei with other heavy multiple-charged ions,
at the appropriate energy, can lead to their composition
to form new superheavy nuclei, as will be
described in more detail below in the "Transurans" section.
In addition to energy, the result of
a nuclear reaction depends on the collision parameter b
of nuclei. With a collision parameter of several tens of
femtometers, a distant collision occurs, the nuclei
flying around each other deviated by elastic (Rutheford)
scattering, and their Coulomb excitation can occur. With the
impact parameter of the fm units, a peripheral collision
occurs, accompanied by scattering, with the possibility of direct
reactions of peripheral nucleons (such as nucleon entrainment).
At b »1
fm there is a tight "sliding" collision,
with deeply inelastic scattering of the cores, with the
possibility of partial fusion or fragmentation. At almost central
collision, b <1 fm, at suitable energy the nuclei may
fuse, at high energies their fragmentation or breakage, at very
high energies even form a quark-gluon plasma, the
hadronization of which produces a large number of secondary
particles (see below).
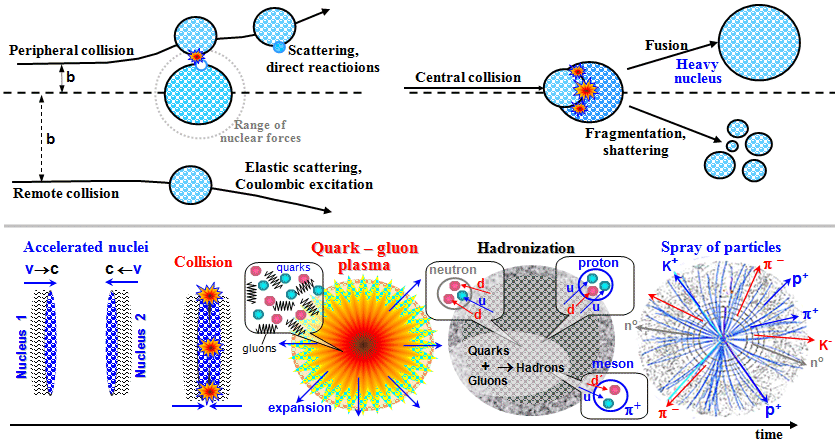
Nuclear reactions in collisions of two heavy atomic nuclei.
Above: When two nuclei collide at low or medium
energies, their electrical (Coulombic) scattering, inelastic
scattering with direct interactions by nucleon transfer or
ejection, fragmentation or composition (fusion) of the nuclei can
occur to form a new heavy nucleus.
Bottom: During a high-energy collision
of two nuclei, a quark-gluon plasma is formed for a short time,
followed by hadronization.
High-energy
collisions of heavier atomic nuclei. Quark-gluon plasma.
We have shown above how nuclear reactions
leading to the formation of new nuclei - nuclear
transmutations, accompanied by the emission of nucleons,
gamma photons, or a -particles. At
energies of hundreds of MeVs to units of GeV, the nuclei
then fragment into individual nucleons or
nuclear fragments, often with the formation of
p- mesons in nucleon-nucleon interactions.
If atomic nuclei collide with the
very high kinetic energies of many GeVs
or TeVs - these are ultrarelativistic nuclear
collisions, nuclei is not enough time to enter into a nuclear
reaction or fragment, but fundamentally new phenomena occur. The
nucleons in the colliding nuclei penetrate each other and
"melt" into a mixture of free-moving quarks and gluons
*): the so-called quark-gluon plasma is formed.
This state lasts only a small moment, about 10-22
seconds, because when the energy of quarks and gluons decreases (during "cooling" due to thermodynamic
expansion), free quarks under the action of gluons
"connect" again to hadrons - nucleons and mesons,
quark-gluon plasma will be hadronized. A spray
of a large number of p- mesons,
protons and neutrons, K-mesons and other particles, as well as
their antiparticles, then flies out of the interaction site.
*) Under normal conditions, quarks are perfectly
"trapped" in hadrons, here in protons and neutrons,
under the action of gluons. When the energy density exceeds about
1 GeV/fm3,
which corresponds to a temperature higher than about 2.1012 °K (temperature one
hundred thousand times higher than inside the Sun!) and occurs at
energy higher than 170 MeV, however, there is a local release
of quarks from hadrons. The quarks and gluons then move freely
for a short time in a mixture called quark-gluon plasma.
In the case of high-energy
(ultrarelativistic) collisions of atomic nuclei, the kinematic
and dynamic effects of the special theory of relativity
are significantly manifested . An interesting phenomenon is the relativistic
contraction, in which the longitudinal dimension of the
cores moving at velocity v is effectively shortened by the
Lorentz factor g = (1-v2/c2)
-1/2. At high energies, where the velocities are very close
to the speed of light, the longitudinal dimensions of the heavy
core relative to the observer at rest can be shortened more than
1000 times! The interaction of the two nuclei then resembles the
collision of two thin disks rather than two
spheres. At these high energies, the effective wavelengths of the
nucleons forming the nucleus are also shortened. This increases
the influence of the details of the structure of the nucleus and
the structure of nucleons - the density distribution of gluons
and quarks. In frontal collisions (central, b »0 fm) most
nucleons in both nuclei succumb to this process, in non-central
collisions only a certain part that collides; the other nucleons
continue to fly as nuclear fragments.
Due to high instability
we cannot analyze the quark-gluon plasma directly, but only
additionally using particles emitted after its hadronization.
Thus, the detectors surrounding the site of the heavy nucleus
collision register only "ordinary" hadrons and other
common particles (photons, electrons, positrons, muons),
regardless of whether or not the quark-gluon plasma was formed
during the collision. To demonstrate the formation of a
quark-gluon plasma, a careful analysis of the number of different
species of flying hadrons and their momentum spectrum needs to be
performed, that could be explained by the presence of free-moving
quarks in the initial phase of the interaction.
The formation of quark-gluon plasma
can be studied on large accelerators using heavy nuclei
("ions"), accelerated to energies of hundreds of GeV
and higher, especially in the opposite direction beams (§1.5, part "Charged
Particle Accelerators", section
"Colliders"). The observation of quark-gluon plasma was first
reported at the CERN SPS in 2000. However, its properties were
analyzed latter at the RHIC (Relativistic Heavy Ion Collider)
accelerator. Research is now continuing at the LHC large
accelerator at CERN, where collisions of lead nuclei with
energies greater than 2 TeV/(nucleon pair) are detected by the ALICE detection system (see §1.5, section "Large
accelerators"). It turns
out that quark-gluon plasma immediately after its formation
behaves as an almost ideal liquid with low
viscosity. From the point of view of particle physics, the
quark-gluon plasma is discussed in §1.5, passage "Quark-gluon
plasma - "5th state of matter"".
Electron-
induced reactions
Electrons do not carry a strong interaction, so that in general
their interaction with nuclei is not significant (of course with the exception of the electrical bonding
of the envelope electrons, forming the structure of the atoms). Under normal circumstances, the nucleus is part of the
atom. At lower energies of the incident electrons, the nucleus is
relatively effectively shielded from them by the repulsive
electrical forces of the electrons of the atomic shell, so that
the bombarding electrons are usually scattered and do not
penetrate the nucleus. When bombarding atomic nuclei with
accelerated electrons, their elastic and inelastic scattering
(with the formation of bremsstrahlung radiation) and Coulomb excitation
of atomic nuclei occur. At high energies in the order of hundreds
of MeV to GeV, the Broglie wavelength of electrons is less than
the effective dimensions of the nucleons, and such fast electrons
penetrate the nuclei, where they can induce nuclear reactions.
..........
Interesting reaction that
may occur during bombardment of nuclei with accelerated
electrons, is called inverse b -decay
- electron penetrates into the core and then is combined with a
proton to form a neutron and emit a neutrino: e- + p+ ® no + n'e. From the point of view of the bombarded core, this
manifests itself as a reaction: e+ + NXZ ® NYZ-1+ n + g. This process
takes place through a weak interaction and its effective cross
section is very small, so it is practically not used in
laboratory conditions. However, it has important astrophysical
significance in the final stages of the life of material stars,
where it leads to a supernova explosion and the
formation of a neutron star - see §4.2 "Final stages of stellar
evolution. Gravitational collapse", section "Supernova
explosion, neutron stars, pulsars" book "Gravity, Black Holes and the Physics of
Spacetime").
Reactions
induced by g -radiation - photonuclear reactions
Even g- radiation
does not show a strong interaction, so it interacts with atomic
nuclei indirectly, through electromagnetic action. At low and
medium energies of the order of MeV units, elastic
scattering (classical Thomson or Compton scattering) of
photons g on nuclei occurs and inelastic scattering
causing an excited state of the target nucleus (followed by deexcitation of gamma radiation emissions). A special case is the resonant nuclear fluorescence of
gamma radiation - the Mösbauer effect (described in more detail in §1.6 "Ionizing
radiation", passage "Interaction
of gamma radiation").
If the quantum radiation g has a sufficiently high
energy, greater than the binding energy of the nucleons
in the target nucleus (at least about 2.5 MeV), they can be
absorbed and induce a nuclear reaction in the
nucleus, in which a neutron or proton is ejected
from the nucleus: photonuclear reactions (g, n), (g, p); at
sufficiently high energies g or even more particles: (g, 2n), (g, np), (g, 2p), (g, a). The simplest
photonuclear reaction is the ejection of a neutron from the
deuterium nucleus g + 2H1 ® p + n (ie its fission
into a proton and neutron), which has a threshold energy of 2.23
MeV. For heavier nuclei, a substantially higher radiation energy g is usually required to
produce a photonuclear reaction. The resulting nucleus after the
photonuclear reaction can be radioactive - we say that the
so-called gamma-activation occurs.
If the energy of the
radiation g causing the photonuclear reaction is only slightly
higher than the threshold energy (given by the binding energy of
the nucleons), the reaction proceeds through a compound nucleus,
and at higher energies by a direct process.
When
irradiating heavy nuclei in the uranium and transuranic region
(such as 235,238U) with hard radiation g of energy higher than 15MeV,
may cause photofission such nuclei into two
fragments - medium-heavy nuclei from the middle of the Mendeleev
table, similar to their fission by spontaneous or neutron action.
At very high energies of gamma
radiation, exceeding »150 MeV, new elementary particles, such
as p -mesons
, are already produced, at even higher energies also K-mesons and
hyperons (as mentioned in more detail in
§1.5 "Elementary particles and accelerators", section "Interaction of elementary particles", passage Formation of new particles
during interactions").
Fission and fusion of atomic
nuclei. Nuclear energy.
General possibilities of obtaining energy from matter
Energy is
contained in mass - substance
in nature. The maximum amount of energy E that can in
principle be obtained from matter is given by the Einstein
relation of the equivalence of mass and energy E = m.c2, where m is
the mass and c is the speed of light. However, the actual
practically available amount of energy is many times (by
many orders of magnitude) smaller.
The following figure shows the ways in which energy
can be obtained from matter :
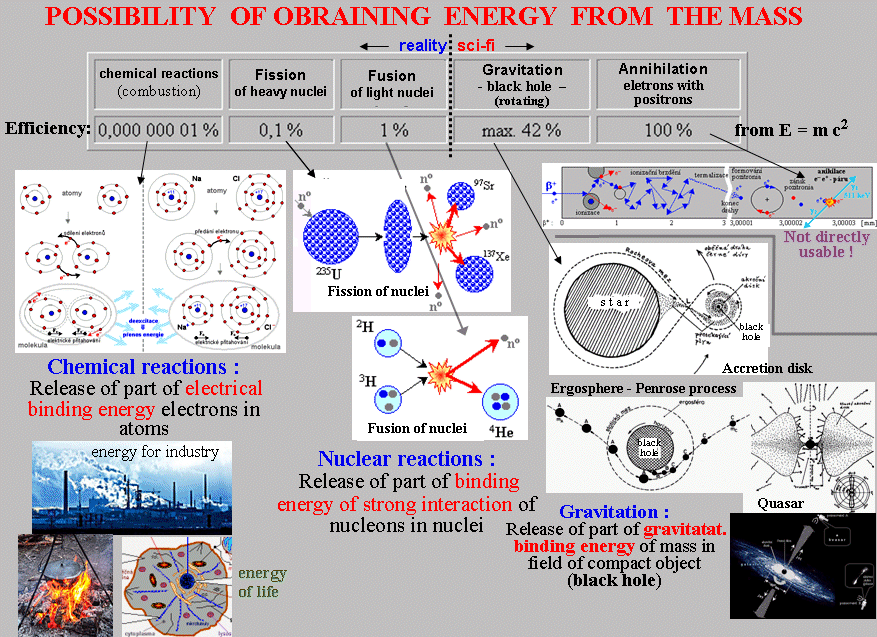
Chemical reactions
Here on Earth, the most common way to obtain energy from matter
is through chemical reactions between atoms, in
which part of the electrical binding energy of
the envelope electrons in the atoms is released . Besides the
energy recovered by living organisms from food, it is
often burning - combining a suitable fuel
with oxygen. However, the binding energy of electrons in atoms is
relatively low, so the efficiency is only 0.000 000 01 % m.c2 (from the maximum possible energy by
Einstein relation E = mc2). Suitable chemical fuels
containing carbon and hydrogen - and atmospheric oxygen
however, there is enough, so that even this negligible efficiency
was until recently sufficient to cover common needs. However,
with industrial development, energy consumption increases, fossil
fuels are depleted and the chemical method will no longer
be sufficient (cf. also below the
reflection "Energy-life-society").
Nuclear reactions
The perspective here is nuclear reactions, in
which part of the binding energy of a strong interaction
of nucleons in atomic nuclei is released. This nuclear
energy is many orders of magnitude larger
than chemical energy. During the fission of
heavy nuclei (eg uranium) about 0.1% mc2 is released, during merging (fusion)
of light nuclei then even higher energy approx. 1% mc2. Both of these
methods will be discussed in detail below.
Gravity, antimatter ?
Theoretically, there are two other more efficient
ways that are, however, in the foreseeable future (and perphas forever?) only at the
level of science fiction :
1. Gravity - the release of the gravitational
binding energy of matter in the field very strongly
graviting object, black holes where
theoretically it can reach a maximum efficiency of up to 42% ( Gravity4-8.htm ). However, we
have no available black hole; even if
we had it, we will not have technologies in the foreseeable
future that would be able to release and use this energy.
2. Annihilation of electrons with positrons, in
which 100% of their mass is converted into gamma radiation (it is discussed in §1.5, passage "Antiparticles,
antimatter, antisworlds"). However, we do not have available the
antimatter and the energy from annihilation is
almost impossible to effectively exploit (discussed in the passage "Antimatter - a potential source of
energy?" §1.5).
Most technologies for obtaining energy from matter are
based on the consumption of fossil fuels that
originated on Earth in the distant past and are not
renewed, either at all, or much
slower than their consumption. They are therefore in danger of
being exhausted. This applies in particular to
coal, oil and natural gas reserves; these fuels are also ecologically
problematic - they contaminate the environment with compounds of
sulfur, nitrogen, carbon dioxide...
Energy
of atomic nuclei
Nucleons in atomic nuclei are strongly bound by nuclear
forces, which is associated with considerable potential binding
energy Eb. It is the energy needed to completely "break
down" the nucleus into individual nucleons, or vice versa,
the energy that is released when the nucleus is
"assembled" from these nucleons. Due to the equivalence
of mass and energy (expressed by the known Einstein relation E =
mc2)
results in the total mass of the nucleus mZ,
N being less than
the sum of the masses of its free nucleons Z.m p + (N.Z).mn. This difference
between the mass of free nucleons and the actual mass of the
nucleus: Dm = Z.mp + (N.Z).mn - mZ,N is called the mass
defect and is related to the total binding energy of the
nucleus by the relation Eb = Dm.c2. The total binding energy of the nucleus Eb increases with the number of nucleons, but for the
stability of the nucleus and the energy balance in nuclear
transmutation, it is more important the mean binding energy per one
nucleon: Eb/N (characterize also sometimes
called. packing factor, mentioned in §1.1 passage
"binding energy of the nucleus") .
For different atomic nuclei, this binding energy per nucleon is
different, as can be seen from Fig.1.3.3. For light elements,
this binding energy increases with the nucleon number, with a few
fluctuations for the lightest elements, nuclei with an even
number of protons and neutrons show higher stability. The
combination of two protons and twoo neutrons forming the nucleus
of helium 4He
is significantly more stable. Above N »20 the growth slows down
and a slow maximum is reached for the elements of the iron group
(chromium, manganese, iron, nickel, copper). For nuclei heavier
than iron, the binding energy of the nucleon gradually decreases
again; this is due to the fact that for large nuclei, in addition
to the attractive short-range nuclear forces, an electric
repulsive force between protons is increasingly being applied.
In small
nuclei, nucleons bind by nuclear forces to a small number of
"neighbors", so they have a relatively low binding
energy per nucleon. As the volume of the nucleus increases, the
inner nucleons bind efficiently to about 12 surrounding
"neighbors", the proportion of surface nucleons
decreases, and the binding energy is established at about 8
MeV/nucleon. The maximum of 8.8 MeV/nucleon is for the iron
nucleus 56Fe,
for larger nuclei, the binding energy per nucleon decreases
slightly due to increasing electrostatic repulsion of protons.

Fig.1.3.3. Dependence of the mean binding energy Eb/one nucleon on the
nucleon number N of the nucleus. In the initial part of
the graph, the scale on the horizontal axis is slightly stretched
to better see the differences in binding energy for the lightest
nuclei. The right part schematically shows both ways of releasing
the binding energy: the splitting of the heavy nucleus and the
merging of the two light nuclei.
By nuclear energy we mean the binding energy of nucleons in nuclei. From the shape of the binding energy curve in Fig.1.3.3 it follows that there are two basic possibilities of effective energy release during nuclear transformations :
In both of these processes, the
nucleons in the resulting nuclei have a greater binding
energy than in the initial nuclei, and the difference
between these binding energies is released - we obtain nuclear
energy.
The third way of releasing nuclear
energy is nuclear decay, where a nucleus with a
slightly different proton number is spontaneously formed from the
original nucleus - by radioactive transformation of
nuclei. However, the amount of energy released here is relatively
small. Nevertheless, this method can be
advantageously used where low energy output is
sufficient, especially in low-current electronics. A device that
uses energy from the decay of radioactive isotopes to produce
electricity is called nuclear battery, radioisotope
generator, or radionuclide volta cell :
Radionuclide Volta Cells ("Atomic"
Batteries)
As will be discussed in detail below, on a
macroscopic scale, we commonly obtain electrical energy from
nuclear energy through thermal energy. It would certainly be
advantageous to convert nuclear energy into electrical energy directly.
We cannot do this in the process of fission or fusion, but in the
case of nuclear radioactive transformations we can do it in part.
Electrically charged particles, which are formed during the
transformation of radioactive nuclei and which fly out of them,
actually represent an electric current. Although this electric
current is very weak, electric voltage and current can be excited
by suitable capture of flying particles - an electric
source can be created on the power of the order of
milliwatts to watts (depending on the activity of the
radionuclide and the performance of electrical conversion). A
device in which electrical energy is generated or accumulated by
the action of radiation from radioactive substances is called a radioisotope
battery (inaccurately also an
"atomic battery"), or a nuclear
battery. Since beta electrons are the
most suitable for direct excitation of electricity (alpha-particles have a short range in matter), we also encounter the name beta-volta cells.
Tritium 3H currently appears to be the most
suitable excitation radionuclide (§1.4,
passage "Hydrogen - 3H"), strontium-yttrium-90,
promethuim 147Pm, plutonium 238Pu or americium 141Am are also used. Radionuclides with low-energy beta- -radiation have the
advantage that no shielding is required (radiation
is absorbed inside and in the battery case) and
there is no radiation in the vicinity.
In principle, it is possible to construct
four types of radionuclide electric batteries, which use
different mechanisms of interaction of nuclear radiation with
matter, in which electric voltage and current are generated :
¨ Direct
excitation
of electric voltage by charged particles emitted during
radioactive transformations. Charged beta or alpha particles are
captured on suitably modified electrodes, where
they transmit their charge and kinetic energy; these electrodes
are thus a source of electrical voltage and current that can flow
through the external circuit. If the electrodes are perfectly
insulated, they function as a capacitor and can generate high
voltages of many kV (due to the
high kinetic energy of charged particles b, a emitted during
radioactive conversion), but they are able
to supply only a very small current and el. power - depending on
the activity of the radiator.
¨ Ionisation
radioisop batteries ,
with a gas filling in which nuclear radiation causes ionization.
Between two electrodes with different contact potential
is a gas charge in which ionizing radiation of an exciting
radionuclide causes the formation of oppositely charged ions. Due
to the different contact potential of the electrodes, the ions
move to the opposite electrodes, where they discharge
and drive an electric current in the outer circuit between the
two electrodes. Argon with the addition of the excitation
radioisotope tritium 3H is suitable as a gas filling.
¨ Semiconductor radionuclide
batteries ,
using the action of ionizing radiation on the transitions
p-n of a suitable semiconductor. Charged particles
emitted by a radionuclide are not direct carriers of electricity
here, but only their energy is used to create
el. current in the semiconductor material. Due to the action of
ionizing radiation, electron-hole pairs are
formed in the semiconductor. An electromotive force then arises
at the p-n junction. The desired effect can also be achieved by
combining a emitter and a semiconductor with a suitable scintillator:
nuclear radiation first interacts with the scintillator material
in which it induces light radiation, and only then excites the
p-n transition; it is similar to a conventional photovoltaic
cell. Semiconductor beta-volta cells excited by tritium 3H appear to be the
most promising.
¨ Thermoelectric
radioisotope batteries
(also called radioisotope
thermoelectric generators - abbreviation RTG ) are made up of common thermocouples
heated by heat *) generated by radioactivity (incl. absorption of
nuclear radiation). This creates an electromotive force,
providing an electric current to the external circuit. It is
possible to use beta and alpha (or gamma) radioactivity here, but
the efficiency is relatively low (approx. 0.2%). In practice, the
plutonium isotope 238Pu is often used for long-term thermoelectric batteries
(eg pacemakers, space probes), (a -radioaktivity,
half-life 87.7 years, has a heat capacity of 0.54 W per 1 gram).
*) This type of nuclear battery actually
gives electricity through thermal energy, only in a different way
than nuclear power plants.
Radionuclide electric batteries have (unlike
nuclear reactors) only very little power. Their energy
efficiency or profitability cannot be spoken of at all:
many times ( many millions of times!) more energy is
used to produce the relevant radioactive preparation than a
radioisotope battery supplies during its entire operation.
However, their advantages are small
(often miniature) dimensions, long service life, mechanical and
temperature resistance. Unlike electrochemical batteries, they
work even at very low temperatures, so they can be used to
advantage in rockets and artificial satellites launched into
space.
Fission of atomic nuclei
In §1.1, the section on the structure of the atomic nucleus ("Structure of the
nucleus"), we mentioned the strong nuclear interactions
holding the nucleus together against repulsive electrical forces
between protons. An important feature of these strong
interactions is their short range of only »10-13 cm. Nuclear forces
appear to be saturated consisting in that each nucleon
attracts strongly only with its nearest neighbors. This property
means that it is not possible to "fold" a stable
nucleus with an arbitrarily large number of nucleons - in large
nuclei, the strong interaction "no longer" reaches
sufficiently from the inside of the nucleus to the peripheral
parts. All nuclei heavier than bismuth are radioactive, mostly
alpha...
This reduced stability of heavy
atomic nuclei is manifested in a specific way by the interaction
of neutrons with these nuclei, which are significantly different
from the usual reactions (n, g), (n, p) , (n, a), etc., occurring in lighter nuclei: often a new
phenomenon occurs for slow neutrons - the fission of
atomic nuclei.
Fissionable
and fissile nuclides
From a purely theoretical point of view, each larger atomic
nucleus can in principle be split into two lighter nuclei by
means of suitable nuclear reactions - by bombarding particles
accelerated to the required energies.
However, only such heavy nuclei
(N>230) are interesting for nuclear technology, which
can be cleaved by neutron absorption (fast or
slow), while nuclear energy is released - the
difference in binding energy / 1 nucleon between lighter and
heavier nuclei. Such nuclei are called fissionable
here. These are, for example, cores 232Th, 233,235,238U, 239Pu, event. heavier transurans, which are, however,
difficult to obtain in larger quantities.
Among these fissionable nuclei, such
nuclei occupy a prominent position, which cleaves "very
willingly" by absorbing even a slow neutron, and during
fission, at least two more neutrons are released, capable of
initiating the fission of other nuclei. They are called fissile
nuclides. In a sufficiently large so-called critical amount
in a suitable configuration, these fissile nuclides are able to
self-sustain a chain fission reaction. The most
commonly used nuclides of this species are uranium 235U and plutonium 239Pu. Whether a
fissionable nuclide will also behave as fissile at the same time,
it decides energy balance of binding energy during neutron
capture, as discussed below in the section"Fission by slow and fast neutrons".
In fields
other than nuclear technology, in general nuclear physics, the
names "fissionable - fissile" are usually not
recognized.
The course of
nuclear fission
We will show the fission of atomic nuclei on a typical example of
235U. When
a slow neutron enters this nucleus, the uranium nucleus splits
into two medium-heavy fragments F1 and F2 *),
emitting 2 or 3 neutrons: 235U + no ® F1 + F2 + (2 -3) no + Q (energy, includes g). The energy balance of fission and the properties of
the fragments will be mentioned below.
*) In addition to the usual binary fission, there is also a
relatively rare type - the so-called ternary fission
(0.2-0.3% of cases), in which the heavy nucleus splits into three
fragments. Two of these fragments are medium-heavy nuclei from
the middle of the periodic table, the third may be a very light
nucleus - helium 4He, tritium 3H, 5He is also observed (which decays
to 6 Li
with a half-life of about 0.8 s.).
We imagine the
mechanism of fission according to the drop model of the
nucleus in the following stages: By capturing the neutron in
the 235U
nucleus, its excitation occurs - for a short
time, the 236U* core is
formed, which is brought into oscillation. As a
result of these oscillations, the originally spherical shape of
the core deforms to an elliptical, at the ends
of which repulsive protons collect. The repulsive electrical
force of the protons overcomes the strong short-range
interaction, the nucleus narrows and constricts in the middle,
until the binding energy is overcome and the nucleus splits
into two fragments, that will fly away by repulsive Coulomb
forces and take about 90% of the energy released. Each of these
fragments sends a "surplus" neutron *)
very quickly, sometimes 2 neutrons - these are neutrons that
remain in a "strangled" place and after the nucleus
ruptures, they explode into the environment. Upon deexcitation of
their excited levels, fission fragments also emit gamma
radiation (referred to as "instantaneous", because it
occurs during the fission process - in
contrast to the subsequent radiation g, generated latter
during the radioactive transformations of fission products; this
radiation can be "delayed" in very different ways, from
microseconds to millions of years, depending on the half-lives of
the radioactive fission products).
*) Neutrons released immediately during
fission are called instantaneous neutrons; there
are about 99% of them and their energy ranges from 0.025 eV to
about 10 MeV. However, during fission reactions, so-called delayed
neutrons are also formed in an amount of about 1% (with
energies in the range of about 0.2-0.6 MeV), originating in
radioactive fragments of fission with an excess of neutrons,
which are removed either by b decay or, especially when in a highly excited state, neutron
emission. These neutrons are emitted with a delay of up to a few
seconds (the mean time of this neutron delay is about 0.1 s). An
example is the fission of a uranium nucleus 235U ® 87Br + 147La + 2n, while the
bromine nucleus 87 remains in a state with high excitation energy
after fission, by b -decay is transformed into a highly excited 87Kr* nucleus , which
changes to a stable neutron emission core 86Kr (the competitive
reaction is its b -conversion to 87Sr). Another cause of delayed neutrons is, for example,
the isotope iodine 137I. Delayed neutrons are of great practical importance
for the dynamics and control of the fission reaction in nuclear
reactors, as will be mentioned below.
Heavy nuclei
(such as 235U) have a higher neutron to proton ratio for their
(relative) stability than stable medium-heavy nuclei. Thus, the
medium - heavy nuclei that are formed during fission have a
significant excess of neutrons, which are
"removed" by several b- -radioactive transformations until stable nuclei are
formed. This produces "beta" electrons and electron
(anti) neutrinos, and gamma radiation during deexcitation of
nuclear levels. This subsequent radiation a and b it can be
"delayed" very differently from the act of fission,
from microseconds to millions of years, depending on the
half-lives of radioactive fission products.
Fission
by slow and fast neutrons
As mentioned above, the initial phase of the mechanism of fission
of a heavy nucleus is its excitation, for which
the necessary activation energy must be supplied to the nucleus
*). The magnitude of this energy (and the appropriate mechanism
for its delivery) depends on the size of the nucleus and the
configuration of the energy levels of the nucleons in the
nucleus; this is explained by the shell and drip model of the
nucleus (in the equation for the binding
energy, the even-odd target nuclei 235U92, 233U92, 239Pu94 have a spin element equal to zero, while the even-even
nuclei 232Th90, 238U92 have a positive spin
member and the threshold energy must be exceeded to perform the
fission).
*) A certain exception is the spontaneous
fission of heavy nuclei without the participation of
neutrons, which can be caused by internal quantum fluctuations of
oscillations in the nucleus. Here too, however, it appears that
excited heavy nuclei are much more easily subject to spontaneous
cleavage (this is a serious problem in the formation and
detection of the heaviest transurans, as will be discussed below
- the "Transurans" section).
For odd
isotopes of heavy nuclei (such as 235U, 233U, 239Pu), it is sufficient to capture a slow neutron
whose binding energy itself on a shell with an
odd neutron is sufficient to vibrate the nucleus and split it.
When a sufficient number of such nuclei is collected in a certain
compact volume, the so-called critical amount, a chain
fission reaction can be triggered - see below. Such
nuclides are called fissile.
For even isotopes (232Th, 238U, 240Pu), the absorbed
new odd neutron is only weakly bound. The binding energy of a
captured neutron is thus not in itself sufficient for the
necessary oscillation and fission of the nucleus - in order for a
neutron to split such a nucleus, it must also bring a certain kinetic
energy: such nuclei are fissile only by fast
neutrons, are referred to as fissionable. These
nuclei do not undergo a chain fission reaction,
most of the emitted fast neutrons leave the space rapidly without
interaction. In order for these even isotopes 232Th and 238U to be used as
nuclear fuel, they must first be converted to odd-numbered
neutron isotopes that are fissile in a chain reaction. However,
the capture of slow neutrons in 238 U and 232 Th - the so-called propagating
nuclides - causes nuclear reactions which, after a series of
radioactive transformations, eventually result in the formation
of odd plutonium nuclei 239Pu and 233U, which are cleavable by slow neutrons and allow the
formation of a chain fission reaction - see "Propagation
reactors" below.

The efficiency of some neutron nuclear reactions, important for
fission nuclear energy, depending on the kinetic energy of
neutrons.
Left: Effective cross section of
fission of three important heavy nuclides depending on the energy
of bombarding neutrons. Right:
Effective cross section of the formation of fission nuclides 239Pu and 233U by bombarding
propagating materials 238U and 232Th with neutrons of different energies.
Note: The complex
course of the effective cross section in the resonance region was
measured using highly sophisticated and demanding experimental
methods. However, in the actual course of nuclear chain
reactions, where fission neutrons have a continuous spectrum with
a wide range of energies, these details do not manifest
themselves. Here, a smooth (strongly smoothed) curve of effective
cross sections is applied.
For each nuclear reaction, the
dependence of its effective cross section (defined above in the section "Effective
cross section of nuclear reactions") on the energy of
the bombardment particles is important. From the left part of the
figure we see that the effective cross section for neutrons
induced fission is the largest for all three basic
fission nuclides 235U, 233U, 239Pu for very slow "thermal" neutrons (about 104 barn) - the slower
the neutrons fly, the they are more likely to penetrate the
nuclei. With increasing kinetic energy of neutrons En (ie neutron velocity
vn) the
effective cross section first decreases monotonically,
approximately according to the law 1/vn
(faster neutrons remain in the field of nuclear forces for a
shorter time, so the probability of a nuclear reaction taking
place decreases). In the region of slow
neutrons of about 10-6 -10-3 keV, the energy dependence of the effective cross
section shows a large number of sharp resonant maxima
and minima (related to the occupancy of
discrete energy levels of nucleons in the nucleus), after which for higher neutron energies the effective
fission cross section stabilizes on the geometric cross section
of core approx. 1 barn. For the 238U and thorium 232Th, a "reasonable" effective fission cross
section appears only for neutrons with energies > 1MeV - these
nuclei are fissile only by fast neutrons.
However, 238U and 232Th nuclei can transmute to 239U and 233Th by neutron absorption - by reaction (n, g) with effective
cross sections according to the right part of the figure,
followed by double beta-radioactive conversion to fission
nuclides 239Pu and 233U. This is used by the so-called "Propagation
reactors" described below.
Energy
balance of fission
The energy Q released during the
fission of heavy uranium nuclei is about 200 MeV. This relatively
large released energy is due to the fact that the binding energy
per nucleon in the region of medium-heavy F1,2
fragments is about 8.4 MeV/nucleon, while
in the uranium nucleus it is about 7.5 MeV/nucleon, ie about 0.9
MeV/nucleon smaller; by multiplying this difference by the number
of uranium nucleons, we get a total released energy Q » 0.9 . 235 @ 212 MeV. The
actual value of the energy released is given by the statistical
average of about 30 cleavage procedures, that occur with varying
probabilities. Most of the released energy Q is carried away by
the nuclei (fragments) F1,2 , whose kinetic energy averages about 165 MeV. Another
part of energy - about 20 MeV - carries g radiation (of which a smaller part of immediate gamma radiation, a
larger part of gamma radiation caused by deexcitation of excited
levels during radioactivity of chips), then
radiation b (approx. 8MeV), neutrons (approx. 6MeV) and flying away
neutrinos (approx. 6MeV - but they will fly
away without use...).
By nuclear fission we can get about
3,000,000 times more energy per unit of mass than by burning
fossil fuels (to produce 100 GJ of thermal energy we have to burn
about 3 tons of coal, or to split about 1 gram of uranium). This high
energy efficiency is the main reason for the development
of nuclear energy using fission
nuclear reactors. Even higher energy
efficiency is expected from thermonuclear fusion, which
is discussed in more detail below in the section "Fusion
of atomic nuclei. Thermonuclear reactions".
Fission Products
In the general description of the atomic nucleus fission
reaction, we have not yet specifically specified the resulting F1 and F2 nuclei (called fragments, slags,
chips, or fission products) to which the 235U nucleus cleaves.
Here are two typical examples: 235U92 + 1n0
® 137Ba56 + 97Kr36 + 21n0 + Q, or 235U92 + 1n0
® 97Sr38 + 137Xe54 + 21n0 + Q, which is just an example of about 30 other more
common combinations of F1 and F2 fragments.
Combinations of F1 fragments
with nucleon numbers 80 to 110 (centered around N = 95) and F2 fragments with nucleon numbers
125 to 155 (centered around N = 137) give the most probable cases
of cleavage. The most common cleavage products of F1,2 are: 137Cs, 93Zr, 99Tc, 90Sr, 131I, 137Xe, .... The curve of the dependence of the occurrence
of fission products on the nucleon number has a characteristic two-peak
shape with the centers of the peaks in the values of
nucleon numbers 95 and 137. From the physical balance of fission
it follows, that the total sum of the yields for all fission
radionuclides (over all nucleon numbers N of fragments) is
equal to 200% .

Graphical plot of the dependence of the proportion of fission
products (% yield per 1 fission) on the nucleon number in the
fission of uranium-235, plutonium-239 and uranium-233 nuclei with
the participation of thermal neutrons. Some of the more important
nuclides formed by fission are indicated by red circles at
positions corresponding to the cleavage yield of the most common 235U fissile material .
For other
fissile materials, 239Pu and 233U, the two-peak dependence of the yield of fission
products on the nucleon number differs only slightly from 235U. The peak yield
for heavier element isotopes (N = 125-155) is practically the
same, while the peak yield of lighter isotopes (N = 80-110) is
shifts slightly to the left for 233-uranium and slightly to the
right for 239-plutonium.
Because the nuclei formed by fission
are substantially smaller than the original heavy nucleus, the
ratio of the number of neutrons and protons required for the
stability of the nucleus is smaller than in the original nuclear
mass of the heavy nucleus. Thus, fission products have an excess
of neutrons. Most fission products are therefore radioactive
(most often b-, due to
an excess of neutrons) and further decays on average into 2 to 3
additional daughter isotopes. Some of the more important fission
products resulting from the fission of 235-uranium, 239-plutonium
and 233-uranium nuclei and their daughter nuclides are listed in
the following table :
| Nuclides | Yield
[% / cleavage] 235 U 239 Pu 233 U |
Half-time | Radioactive transformations b - | ||
| 134 Cs | 6.8% | ....... | ....... | 2.06 years | 134 Cs (2.06r) ® 134 Ba (stable) |
| 135 I | 6.3% | ....... | ....... | 6.57 hrs. | 135I(6,7hod.)® 135Xe(9,2hod.)® 135Cs(2,6.106let)® 135Ba(stab.) |
| 93 Zr | 6.4% | 3.9%. | 6.9% | 1.5 . 10 6 y | 93 Zr (1.5.10 6 r) ® 93 Nb (stable) |
| 137 Cs | 6.1% | ....... | ....... | 30.17 years | 137 Cs (30r) ® 137 Ba (stable) |
| 99 Tc | 6.1% | 6.2% | 5.0% | 211 000 y | 99 Tc (2,1.10 5 r) ® 99 Ru (stable) |
| 90 Sr | 5.7% | 2.0% | 6.6% | 28.8 years | 90 Sr (28.8r) ® 90 Y (2.66d) ® 90 Zr (stable) |
| 131 I | 2.8% | ....... | ....... | 8.02 d | 131 I (8d) ® 131 Xe (stable) |
| 147 Pm | 2.3% | .fill in.. | ....... | 2.62 years | ......... |
| 149 Sm | 1.1% | ...... | ....... | stable | - |
| 129 I | 0.7% | 1.4% | 1.6% | 15.7 . 10 6 y | 129 I (15.7 . 10 6 r) ® 129 Xe (stable) |
| 151 Sm | 0.42% | 0.8% | 0.3% | 90 years | ......... |
| 106 Ru | 0.39% | ....... | ....... | 376.3 d | ......... |
| 85 Cr | 0.27% | ....... | ....... | 10.8 y | .....fill in.... |
| ......... | ........ | ....... | ....... | ......... | |
| ......... | ........ | ....... | ....... | ......... | |
| ......... | ........ | ....... | ....... | ......... | |
| ......... | ........ | ....... | ....... | ......... | |
| ......... | ........ | ....... | ....... | ....fill in..... | |
| ......... | ........ | ....... | ....... | ......... | |
Note:
Numerical values from the nuclear tables "Lederer,
Hollander, Perlman: Table of Isotopes" and from the tables
"IAEA: Nuclear Data for Safeguards" were used to draw
graphs and create a table. It will be specified according to
other data ...
The summary of
nuclides formed by fission is called a mixture of fission
products. About 60 isobars of various types of nuclei
(mostly with an excess of neutrons) are formed directly during
fission, most of which decay into 2-3 more daughter
radioisotopes. In the fresh fission mixture we can find almost
1300 different radionuclides, most of which have short half-lives
(eg the mentioned
137Xe has a half-life of only 4
minutes, with which it changes to 137Cs); more important are about 180
radionuclides. Short-term radionuclides are not listed in the
graph or table, we consider only half-lives of the order of
hours, days, years and longer. The isotopic composition of the
fission mixture changes significantly over time.
Initially, the specific activity is very high due to radioactive
transformations of short-term radionuclides. Short-term
radionuclides decay rapidly, the specific activity decreases
significantly and after a few days 131I, later 137Cs, 90Sr and others dominate. After many decades, long-lived
radionuclides such as 99Tc, 93Zr, 135Cs persist and smaller amounts of some others. These
radionuclides form a difficult and long-term dangerous component
of spent nuclear fuel, which must therefore be stored for a long
time. An alternative possibility of nuclear transmutation
of long-lived radionuclides is discussed below ("Nuclear waste", transmutation technology).
Chain
Fission Reaction
When a nucleus is split, the neutron that caused the fission
reaction is "consumed", but during the reaction two
more (or three) "2nd generation" neutrons are emitted,
which are in principle capable of inducing the fission of other
nuclei. If this happens, these new neutrons will cause the
fission of two more nuclei to form a total of 4 neutrons, these
will cause further fission, etc. - the number of neutrons in each
"generation" is rapidly multiplied by a geometric
series and the rate of branching nuclear fission reaction increases
avalanche - occurs nuclear chain reaction.
The usability of neutrons for fission is reduced by two competing
processes :
- Neutron
capture in the nuclei of the environment. This can be
either radiation trapping in the fissile material, which leads to
a different nuclear reaction than fission, or neutron trapping in
the non-fissile material of the fuel mixture (especially in the
so-called "neutron poison" - see below).
- Leakage
of neutrons from the reaction volume into the
surrounding space.
Nuclides for Chain Reactions - Fissile and Propagating Materials
In order to maintain a chain fission reaction, it is necessary
that, on average, at least one neutron released during fission
"survives" in the reaction space, enters into the
nucleus of fissile material and induces a new fission reaction.
This can only be done if the nuclides used can be cleaved by very
slow neutrons; this is because most fast
neutrons soon leave the reaction space and are not sufficient to
cause fission (discussed above in the
section "Fission by fast and slow neutrons"). These nuclides are called fissile materials
(....) and only four
are available *) : uranium 235U , plutonium 239Pu
, uranium 233U and possibly plutonium 241Pu.
*) Some higher transurans,
such as americium or californium, also have this property (and even more effectively - less critical amounts) however, due to the complex preparation, they are only
available in small quantities, which does not allow the chain
reaction to operate.
The only
fission material occurring in nature, is uranium 235U.
The other three mentioned fission nuclides must be obtained by
bombarding the so-called propagating materials (....) of uranium 238U and thorium 232Th by neutrons (theoretically
analyzed above in the section "Fission
of atomic nuclei",
passage "Fission by slow and fast neutrons",
practical use is described below in the section "Propagation
reactors"). After neutron absorption, two subsequent beta
transformations take place here, resulting in the cleavable
nuclei of plutonium-239 or uranium-233.
Chain reaction
dynamics
The so-called multiplication factor k is
important for the chain reaction dynamics, which is the ratio of
the number of neutrons of the next generation to the number of
neutrons in the previous generation, and the mean lifetime of
neutrons tn in the reaction
medium, also called the mean neutron cycle time;
it is the time between the next two succesive generations of
neutrons. If at a certain moment n neutrons are present in
the fissile material, then after the time tn their number will be k.n, so the increase in neutrons
during the time tn is k.n-n = n. (k-1).
Thus, the equation dn/dt = n .(k-1)/tn will apply to the rate of change of the number of
neutrons. The solution of this differential equation is the
exponential dependence
n(t)
= no .e [(k-1)
/ tn] .t ,
where no
is the number of neutrons in the initial time t = 0. The dynamics
of the increase or decrease in the number of neutrons, and thus
the running away or slowing down of the fission reaction, is sharper
the more the multiplication factor k is greater
than or less than 1 and the shorter is the mean time of the
neutron cycle tn . For k> 1
the reaction increases, for k
<1 the reaction stops, in the
special case k = 1 the reaction is kept constant.
Note: A chain
nuclear reaction can be compared to a chemical chain
reaction - see §1.1, section "Interaction of atoms".
Critical amount of
fissile material
In order for such a chain reaction to occur, it is necessary to
have a sufficient amount of fissile material
concentrated in a certain volume - at least the so-called critical
amount (weight); with a smaller amount, the vast
majority of neutrons it escape from the
substance (or it is absorbed in another way), before it is enough
to split another nucleus. The critical amount of fissile material
in specific situations depends mainly on three factors :
w Type
of fissile material and its concentration
These must be nuclei cleavable by slow neutrons (235,233U; 239Pu and other
transurans) with a high effective cross section of the
interaction with slow neutrons. The higher the effective neutron
capture cross section, the smaller the critical amount. The
critical amount is inversely proportional to the square of the
density of the fissile material.
w Dimensions and geometric arrangement of
the area containing fissile material
The more compact the geometric arrangement, the smaller the
critical mass. It is the lowest for the arrangement of fissile
material in a spherical volume, where the
highest ratio of volume to surface size (through which neutrons
can escape) is.
w Presence of other substances and
materials capable of absorbing, reflecting or slowing down
neutrons
Substances with a high effective neutron
absorption cross-section significantly increase the
value of critical mass. The presence of substances capable of reflecting
flying neutrons (and thus returning them to the reaction) reduces
critical mass, as do light nuclei capable of slowing down
(moderating) neutrons during elastic reflections - see below
"Nuclear Reactors".
For individual types of fissile
materials, their critical mass mcrit is given for a spherical
homogeneous arrangement (of radius Rcrit) of pure material, eg :
235 U: m crit = 48 kg, R crit = 9 cm ;
239Pu: m crit = 17 kg, R crit = 6 cm ;
233 U: m crit = 16 kg, R crit = 6 cm ;
for some other transurans the critical amount is even smaller (eg 245-curium 12kg, 246-curium 7kg, 251-californium
9kg). Of interest is transuranic americium
242mAm, which of all fissile materials has the
highest effective cross-section of fission by slow neutrons (thousands of barns) and a low
critical mass (approx. 0.1 uranium) - it is considered promising for rocket propulsion in
the future - see below "Nuclear
propulsion of cosmic rocket".
If the fissile material is
surrounded by a substance that reflects neutrons (so-called
reflector or neutron shell), the critical amount decreases
2-3 times. If the concentration of the fissile material is less
than 100%, the critical mass increases significantly,
especially when neutron absorbers are present. For low
concentrations of fissile material, there is usally no critical
amount exists and the chain fission reaction cannot arise
spontaneously; the possibilities of fission reactions even in
such cases, using neutron moderation or ADTT technologies, will
be discussed below.
Storage the
supercritical amount of fissile material is a very delicate
matter. This is because the critical amount for a given (used)
configuration may be exceeded, which would lead to an
avalanche-like chain fission reaction (k> 1) with very dangerous
radiation consequences. Persons at the scene of an
accident would receive very high, often lethal, doses of
radiation (see §5.2 "Biological effects of ionizing radiation"), followed by significant contamination
of the environment with radioactive fission products.
To prevent this, it is necessary to
store the fissile material in an arrangement or containers with
the so-called safety geometry - with the largest
possible surface area in relation to the volume (unlike to a
spherical arrangement, where the opposite is the case), so that
most neutrons easily escape outside the volume of the fissile
material and thus cannot cause further fission.
Preparation
of fissile material
The material capable of a chain fission reaction can be of natural
origin or produced artificially. There
is only one nuclide in nature, directly usable for the chain
fission reaction - uranium 235U. It is contained in uranium ore,
which has the following representation of individual uranium
isotopes: 238U 99.284%, 235U 0.711% and trace amounts 234U (0.005%). The amount of 0.7% fissible 235U in most
technologies is not sufficient to start and
maintain a chain fission reaction. Therefore, it is necessary to
artificially increase its occupancy - to perform the so-called enrichment
of uranium with the isotope 235U.
Uranium enrichment
Uranium enrichment is a
technologically very demanding and expensive process. It cannot
be done purely chemically (all uranium isotopes have almost the
same chemical properties), but it is necessary to use slightly
different physical properties of different uranium isotopes *).
In the first phase, uranium is chemically combined with fluorine
to form hexafluoride gas UF6, which is then separated by repeated
diffusion separation in special separation columns (isotope
diffusion of UF6 gas through porous baffles) or in high-speed
ulracentrifuges (mechanical-gravity separation), which are
arranged in cascades. Slightly different molecular weights of
compounds 235UF6 and 238UF6 are used. The fluoride fraction with a correspondingly
increased content of 235U is then again chemically converted into other suitable
compounds, or metallic enriched uranium.
*) Isotopes of the same element are
characterized by small differences in nuclear, chemical, physical
and partly also chemical properties. These "isotope
phenomena" are due to the different masses of nuclei and
atoms of individual isotopes, which are manifested by subtle
differences in the kinetics of atoms and molecules in mechanical
motion and chemical fusion. They can be used for chemical-isotope
separation of elements and their isotopes.
The laser method of
uranium enrichment is interesting at the stage of development .
It is based on the principle of selective excitation of
atoms in the gaseous state by means of light radiation
of such a wavelength that excitation occurs only in the atoms of
one isotope of the element, while the atoms of the other isotopes
remain in the ground state. The excited atoms can then be
separated electromagnetically or by a suitable chemical reaction.
The mixture of uranium 235 and 238 in the gaseous state is
irradiated with precisely tuned lasers, which excite only
molecules with 235U, which allows their subsequent separation.
Another
fissile material, plutonium 239Pu,
is practically non-existent in nature *), as it has a
significantly shorter half-life than uranium isotopes. However,
it can be produced artificially from uranium 238U by neutron fusion
in a nuclear reactor (see below "Other
fissile materials. Transurans. Propagating reactors.").
*) However, a very small trace amount
(<10-14)
of plutonium occurs in uranium ores, where it is formed from 238U by neutrons
emitted from nuclear reactions (a, n), caused by particles a from the
radioactivity of uranium and its daughter products (decay
series), in light elements contained in uranium ores.
The fissile material, properly
enriched, is then converted into the final chemical and material
form suitable for use in the fuel cells of nuclear reactors (see
below).
Uncontrolled Chain
Reaction - Nuclear Explosion
The nuclear chain reaction can take place either in a controlled
- regulated manner (this will be
discussed below in the "Nuclear Reactors"
section) or in an uncontrolled
- avalanche, which can result in a nuclear explosion.
Fission Nuclear
Weapons
An explosive nuclear chain reaction is the essence of the
criminal misuse of nuclear energy for war purposes in a nuclear
bomb (often also called the "atomic
bomb"), at which
explosion releases a large amount of energy, from a relatively
small amount of nuclear material. Fissile material - uranium 235U
or plutonium 239Pu - is divided into two or more parts
(segments) in the bomb at rest, each of which has a subcritical
amount with its volume. A nuclear explosion is triggered by these
segments rapidly approaching each other by the
explosion of a suitable chemical explosive ("shot"
into each other - "cannon" method in the picture on the
top left), creating a geometry of the supercritical
quantity. In newer types, the supercritical amount is achieved by
compressing the circularly arranged fissile material by chemical
explosion of the outer spherical shell ("implosive"
method, bottom left in the figure).
| Basic principles of construction and operation of fissile and thermonuclear nuclear weapons | ||
 |
||
| Left: Fission nuclear bomb (two different constructions) Right: Thermonuclear bomb |
When a critical amount is reached
quickly, a nuclear explosion occurs immediately,
as a small amount of neutrons, which are always present in the
material (formed by spontaneous fission of
nuclei and cosmic radiation) initiates
an avalanche-like chain reaction *), which in about 10-6 seconds it splits
almost all the nuclei and a large amount of energy
is explosively released (about 1.107 kJ of energy is
released from 1 kg of uranium, which corresponds to the explosion
of about 20,000 tons of the classic trinitrotoluene explosive). In a nuclear bomb, the fission material is surrounded
by a massive envelope, which serves both as a neutron reflector
and, with its mechanical strength, keeps the fission material
together as long as possible so that it is possible to split as
much material as possible at once. During an explosion, the
remainder of the fissile material disperses to a subcritical
amount, thereby stopping the chain fission reaction itself.
*) In pure fissile material, the mean time
of the neutron cycle is very short, tn »10-8 s, so that even at a
slightly supercritical ratio (eg k = 1.2) according to the above
exponential dependence, a single initial neutron will cause in
only 4 ms the emergence of
about 1025 neutrons and the same number of nuclear
fission, which corresponds to the fission of about 50 kg of
uranium in 4 microseconds! The rate of increase of the chain
reaction is therefore extremely high - it has the character of a violent
explosion.
Due to the compression
caused by the (chemical) explosion, the density of the material
increases and the sufficient critical amount of fissile material
is slightly less than the above values for
uranium or plutonium under normal conditions. This required
minimum amount can be further reduced by a suitable envelope
serving as a neutron reflector and by the use of
a neutron initiator - an additional radioisotope
source of neutrons (eg 210Po-beryllium) located in the center of the charge. By
compression, the a-emitter (210Po) and the target material (beryllium) come into close
contact, and the released neutrons serve as a "igniter"
of the fission reaction. The neutron initiator accelerates
the dynamics of the chain reaction, which does not have
to start from a few initial neutrons, but a large number of
neutrons causing a chain reaction are rapidly delivered
throughout the volume of the fissile material. The critical
amount is also reduced by the moderating effect of
substances capable of retarding neutrons (see "Nuclear
reactors" below). By combining different methods, the
smallest critical amount can be achieved for uranium, about 15
kg, for plutonium, about 5 kg.
The explosion
temperature reaches the order of 107
°C and the explosion is accompanied by intense
ionizing radiation and extensive radioactive
contamination with fission products, which multiplies
the destructive and deadly effects of the explosion itself.
"Improvements" to fission nuclear bombs are thermonuclear
weapons (on the right-hand side of
the figure), which use fission as a
"detonator" to trigger an explosive fusion reaction of
deuterium and tritium, releasing significantly more energy (see "Merging atomic nuclei - thermonuclear
fusion" below, passage "Explosive thermonuclear reactions").
The first
fission nuclear bomb was developed in 1942-45 in the nuclear
laboratories in Los Alamos by a group led by R.Oppenheimer *),
was completed and tested in the spring of 1945 as part of the
"Manhattan" project. The legitimate reason for the
accelerated realization of a nuclear weapon was the threat of
Hitler's fascist Germany. However, at the end of World War II, on
August 6 and 9, 1945, two atomic bombs, uranium and plutonium,
were dropped on the Japanese cities of Hiroshima and Nagasaki.
More than 130,000 people, the vast majority of civilians, died in
this war crime (from a military point of
view already nonsensical), and the
consequences of the exposure in tens of thousands of other people
caused later deaths or permanent damage to their health.
Fortunately, nuclear weapons have not been used since then, they
only served as a "deterrent"- perhaps
paradoxically, they contributed to peacekeeping during the "Cold
War" (fissile weapons in 1949 and thermonuclear weapons
in 1953, the Soviet Union also developed).
*) J.R.Oppenheimer,
often referred to as the "father of the atomic bomb",
along with others prominent physics and co-workers, but after
1945 began to publicly highlight the threat of nuclear
weapons (and crime of their use) and called for control
over the development and manufacture of such weapons of
mass destruction and killing. For these peaceful
activities, Oppenheimer was persecuted by the
then US regime (where Cold War activist R.S.Truman ruled) and was
expelled from all leadership positions. He was rehabilitated only
after the accesion of democratic president J.F.Kennedy in 1961.
Nuclear reactors
These are complex devices in which a purposefully regulated
chain fission reaction takes place. In order for a nuclear
fission chain reaction to proceed in an equilibrium
controlled manner, three things must be ensured in
principle :
a) Collect a supercritical amount
of nuclear fission material for a given configuration.
b) Ensure control of the number of
neutrons by means of suitable absorbers and moderation
of neutron energy so that the fission reaction proceeds
with the required intensity. This regulation of the neutron
balance controls the power of the nuclear
reactor.
And, of course, it is necessary to ensure the dissipation
of released energy, which is mainly converted into heat,
or :
c) Ensure cooling of the
reaction zone - removal of heat generated by a suitable cooling
medium (usually water,
sometimes inert gases, occasionally molten salts or metals; these
methods will be specifically discussed below).
The dynamics of cleavage reaction
determines the ratio between the average number
of new neutrons and the number of neutrons consumed for cleaving
(or the ratio between the number of neutrons following
generations and the number of neutrons of the previous
generation) - called neutron multiplication factor k,
also called multiplication factor neutron (has already been used above in the general analysis of
chain fission reaction dynamics). When the
multiplication factor k is less than 1, the reaction ceases,
at k = 1 it is maintained in equilibrium,
at k greater than 1 the number of fissioning nuclei
increases avalanche, until for some reason the
factor k acquires values k <1 (mainly
regulation by neutron absorption); if this
does not happen in time, the reaction will become explosive.
The controlled chain reaction
of nuclear fission (especially 235U) takes place in a complex device called a nuclear
reactor *). First, we present a general description of
the reactor, relating mainly to the classical reactor splitting 235U with slowed
neutrons (Fig.1.3.4); alternative solutions will be listed below.
*) Note: The
first experimental nuclear reactor, the "atomic
milestone" CT-1 composed of uranium, a graphite moderator
and cadmium control rods manually shifted, was built by E.Fermi
and his colleagues in 1942 in the basement under the stands of
the stadium in Chicago.
The control of the number of
neutrons maintaining the fission reaction is carried out in two
stages in a conventional reactor (a minor
third option is mentioned below - "3. Reactor control by
controlled moderation") :
1.
Moderation of neutrons
(lat. Moderari = to moderate, tame, delay, slow down
)
Neutrons emitted during fission, which usually have
relatively high energies (on average about 1.5 MeV), slow
down *) to a "thermal" energy of about
2.5 eV (given by their thermal motion in the substance)
by interaction with substances of low nuclear mass -
so-called moderators, so that these
neutrons remain in the reaction space long enough to
carry out further fission with a sufficiently large
effective cross section.
*) Although the number of neutrons
per fission increases with the energy of the initiating
neutron, with this energy the probability of its
interaction with uranium nuclei decreases much faster.
Fast neutrons, when passing between 235U nuclei, would not have enough time to
effectively enter the nuclei (and thus cause further
fission) - they would usually fly out of the reaction
space. Slow neutrons can be attracted by nuclear forces
during repeated close passages around the nuclei and
effectively penetrate the nuclei - causing further
fission. In addition, when the fissile material is a
mixture of uranium 235 and 238, medium-velocity neutrons
are radiatively trapped by the nuclei 238U and thus
are lost for further reactions, while slow (thermal)
neutrons hardly enter the 238U nuclei, but readily enter the 235U nuclei and
cause fission. Thus, the moderator by slowing down the
neutrons, returns them to the reaction
and thus helps to maintain the chain fission reaction.
Note:
Fast, ie non-slowed (unmoderated) neutrons are used in
promising FBR reactors with 238U, see below "Other fissile materials.
Transmutation. Propagating reactors.".
Suitable
moderators are those whose nuclei have a high effective
cross section for elastic neutron collisions, with a
sufficiently large loss of neutron energy per collision.
Thus, substances containing light nuclei
are effective moderators, because according to the law of
conservation of momentum and energy, the greatest
momentum and energy is transferred during the elastic
collision of a neutron with a light nucleus *).
*) Conversely, when a neutron
collides with a heavy nucleus, a reflection occurs and
the kinetic energy of the neutron changes little. We can
imagine this in the analogy of a ping pong ball as a
neutron, another ball as a light core and a billiard ball
as a heavy core. When a flying light ball hits another
(standing) light ball, more than half of the energy is
transferred, while when it hits a heavy billiard ball,
this billiard ball barely moves out of place and the ping
pong ball bounces off with almost its original value of
kinetic energy.
The most effective moderator is
therefore hydrogen *), which is
abundantly present in the water.
*) However, due to its low proton density, hydrogen gas
moderates only very weakly. Hydrogen bound in water
H2O or in heavy water D2O has a more effective
moderating ability. Even more effective is the chemical
bonding of hydrogen directly to the 235U fissile material in the
form of uranium hydride UH3. Due to this moderating effect and the
temperature dependence of the synthesis and decomposition
of UH3, special compact self-regulating reactors can
operate (see "Compact self-regulating reactors" below).
Another
requirement is that this substance absorbs little
neutrons. From these points of view, a suitable moderator
is water or heavy water, carbon
(graphite), beryllium (but not
boron, which effectively absorbs neutrons!).
2.
Neutron absorption
To reach the value of the multiplication factor
k = 1, the excess neutrons (which
would otherwise cause avalanche fission and reactor
failure) must be absorbed
in a suitable absorber - most often boron (in the form of carbide) or
cadmium, which have a high effective
cross section for thermal neutron absorption. The
absorbers are usually made in the form of rods,
which are inserted into the reactor and thus control
the reaction rate: if we want to increase the
number of cleavages, we pull out the rods slightly, to
slow down the reaction we slide the rods inwards.
Neutron detectors are located at
various places around the reactor, and the temperature
and pressure in the active zone are monitored, as well as
the instantaneous thermal output of the reactor. The
intensity of the neutron flux is a sensitive indicator of
the intensity of the fission reaction inside the reactor
core, which allows operatively feedback electronically
control of the absorption rods and thus the reactor
operation (see below "Fission
Reaction Dynamics and Nuclear Reactor Control").
Dissolved boron (boric
acid) is sometimes added to the
cooling water to affect the long-term reactivity as a
neutron absorber, the concentration of which gradually
decreases by dilution for better control during fuel
combustion (see below).
Graphite-boron combination ?
In some graphite-moderated reactors (RBMKs), the
absorption-control rods were provided at the end with a graphite
part (boron at the top,
water gap in the middle, graphite at the bottom) for a more homogeneous neutron flux and smoother
positive or negative regulation during rod extension and
retraction. The graphite part caused
"displacement" of water from the channel as the
rod moved. This should lead to a wider range of
neutron flux control as the insertion of the
graphite part (slightly moderating)
slightly increased reactivity
compared to "empty" channels with water (which easily absorbs neutrons). It was also supposed to slightly increase
the utilization and lower concentration of 235U in fuel
cells. In practice, however, this "trick" was
not very applied, the effect was not significant and
according to some opinions it could cause instability of
regulation in anomalous situations (but
it has not been proven, it is briefly mentioned below in
the section "Chernobyl nuclear reactor accident") ...
3.
Reactor control using controlled moderation
There is another mechanism to control the chain fission
reaction without the need for controlled neutron
absorption. It is a controlled moderation of
neutrons. By increasing and decreasing the
concentration of moderating substance in the active
volume of the reactor, we can increase or decrease the
rate of the fission reaction and thus regulate
the reactor output. Neutron moderation can be
controlled artificially from the outside, but with a
suitable technical construction and material design of
the moderating substance it is even possible to achieve
an autoregulatory function using a negative
temperature coefficient of reactivity. There are
projects of smaller compact reactors based on this
principle - see below "Compact self-regulating reactors".
Note:
After all, the ancient "natural nuclear reactors" mentioned below probably worked on this
principle.
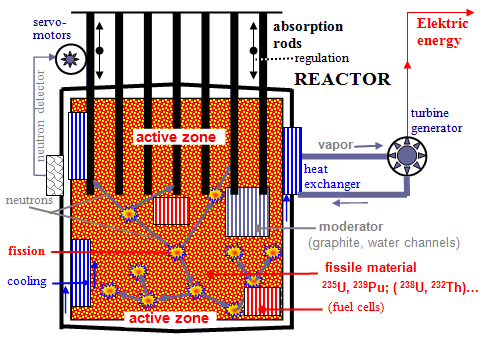 |
Fig.1.3.4. Simplified schematic diagram of a fission nuclear reactor. Note: For the sake of clarity, the structure of the active zone (fuel cells) is not shown in the figure. |
The part of the
reactor in which the fissile material is located and in which the
chain fission reaction takes place is called the active
zone - core. The fissile material
(which is mostly enriched uranium) is stored in the reactor in
the form of a large number of separate and independent so-called fuel
cells, where the fissile material is encapsulated in a
package protected by a suitable surface layer. In water-cooled
reactors, zirconium (niobium-alloyed) is used for
encapsulation, characterized by low neutron absorption (less
uranium enrichment is sufficient); for fast reactors, the coating
tubes are made of stainless steel (alloyed with nickel, chromium,
niobium). There is a moderator between the fuel cells and regulating
absorption rods are also inserted between them (in the pictures, the fuel cells, moderator and cooling
are only sketchily drawn for simplicity, the fissile material is
marked as a granular volume). The core of
the reactor is further surrounded by a so-called reflector
- a layer of suitable material that reflects the escaping
neutrons and returns them partially back to the reaction volume
of the reactor, which somewhat increases the yield of the
reaction. In the reflector, basically the same materials are used
as in the moderator - graphite, heavy water. The inner part, the
primary circuit, of the reactor is stored in a solid reinforced
concrete protective envelope with a hermetic steel lining,
so-called containment (in
sense of : contain , container = closed vessel, box,
case; containment = control, restriction ).
Release and
utilization of nuclear energy from fission reactions
During the chain fission reaction, a considerable amount
of energy is released - nuclei fragments, flying
out with high kinetic energy, quickly slowed down
by the impacts on surrounding atoms and thus transfer their
energy to the material in the form of heat. The
fissile material is therefore heated and must be intensively
cooled by a suitable cooling material (eg water *)
flowing directly around the fuel cells - this is the so-called primary
cooling circuit. In two-circuit systems, the heat from
the primary cooling circuit in the heat exchanger is
transferred to the water of the secondary cooling circuit;
in a nuclear power plant the secondary cooling
circuit is a steam generator, the steam of which
rotates the turbine blades driving the generator
producing the electric current (again, for simplicity, the primary and secondary
circuits are not distinguished in Figures 1.3.4 and 1.3.5).
*) Note:
Water can be advantageously used
both as a moderator and a refrigerant.
The use of water as both a refrigerant and a moderator leads to a
negative reactivity temperature coefficient :
as the temperature increases, the density of water and thus the
proton density decreases, which reduces the moderating effect and
the intensity of the fission reaction. In the event of water
leakage from the primary circuit, the moderating effects are lost
and the fission reaction stops itself ® greater safety against
accident in case of failure. In two-circuit systems, the primary
cooling circuit is hermetically sealed so that its radioactive
water does not mix with the water in the secondary circuit.
Water for cooling and
moderation in the primary circuit of a nuclear reactor should be demineralized
for two reasons: 1. Minerals are excreted from
the water and precipitate on the walls of the cooling tubes,
which reduces the efficiency of heat exchange; chemical reactions
with metal surfaces can cause corrosion. 2.
Intense neutron flux can induce radioactive isotopes by nuclear
reactions with the nuclei of mineral atoms. Boron (boric
acid, approx. 12 g /liter of water) is sometimes added to the
cooling water as a neutron absorber, the concentration of which
is gradually reduced by dilution for better control during fuel
combustion (see below).
Thus, a
nuclear reactor for energy use serves only as a mere "steam
boiler" and further conversion into electrical
energy takes place "old-fashioned"
via a turbine and an electric alternator. Unfortunately, we
cannot directly convert the released nuclear
energy into electricity. Certain hypothetical
possibilities for the direct conversion of particle energy into
electrical energy are outlined below in the passage "Neutron-free fusion reactions
- direct conversion to electricity?"
(however, this is not usable in a fission
nuclear reactor, but perhaps only in future thermonuclear reactors..?..).
The picture on
the left shows a schematic representation of the production of
electrical energy in a power plant (nuclear here), the
transformation of voltage from 6 kV to 220-400 kV, in the middle
is the long-distance line to the consumption area. For
long-distance energy transmission, it is advantageous to
transform to high voltage, where a relatively small current is
sufficient, which minimizes ohmic losses in the line and also
thinner wires are sufficient (mostly Al-Fe ropes with a
cross-section of about 300mm2 are used). However, if the voltage
is too high, about >500kV, losses increase again due to air
ionization and small corona discharges. The picture on the right
shows a schematic representation of the transformation to 22 kV
in a transformer station and finally to 220 V for powering common
electrical appliances at consumers :
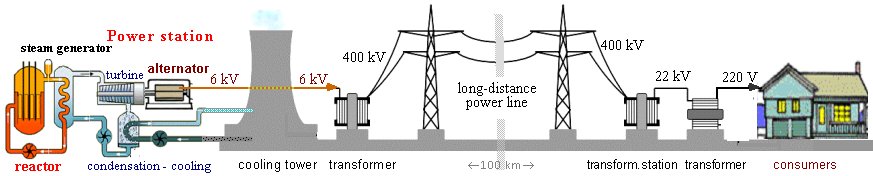
Production
and distribution of electrical energy
Dynamics
of fission reaction and regulation of a nuclear reactor
For the dynamics of fission reaction control in a nuclear
reactor, the speed with which the increase or
decrease in the number of neutrons in individual generations (and
thus the intensity of the fission reaction) reacts to the change
of the multiplication factor k is important. The time
dynamics of the instantaneous neutron flux F, and thus the reaction rate
and the instantaneous reactor power, is given by the exponential
dependence F(t) = Fo.e [(k-1)/tn].t (as derived above in the general analysis
chain reaction), which can be written as F(t) = Fo.e t/T. The time constant
(also called the period ) of the reaction T,
which is the time during which the number of neutrons changes
e-times (ie 2.718 times), is approximately given by the relation
T = tn/(k-1), where tn is average lifetime
(or residence, diffusion) of a thermal neutron in the reaction
medium; in larger reactors it is of the order of 10-3 s. To change the
multiplication factor by eg one hundredth (| k-1 | = 10-2), the decrease or
increase of the reaction would react with a time constant of
approx. T = 10-3/10-2 = 0.1 seconds. With such a small time constant, the
changes in neutron flux would be so abrupt that it would be very
difficult to control the reactor. However, this dynamic applies
to the so-called instantaneous neutrons,
released immediately during fission. However, during fission
reactions, so-called delayed neutrons are also
formed, originating in radioactive fragments of fission with an
excess of neutrons, which they get rid of by b conversion or
neutron emission. These neutrons are emitted with a delay of up
to a few seconds (the mean time of this neutron delay is about
0.1 s). This phenomenon causes the average lifetime of one
generation of neutrons to be significantly longer than the
diffusion time of thermal neutrons, which extends the
effective time constant of the reactor to values of the order of
10 sec.
In conventional reactor designs, the regulation
of the instantaneous reaction rate and thus the reactor power
takes place by means of neutron-absorbing control rods,
driven by electronically controlled servomotors in feedback
with detectors neutron flux. The so-called emergency rods,
which are released by an emergency accident signal and
automatically fall into the active zone by their own weight,
serve for a quick emergency stop of the fission reaction and thus
shutdown of the reactor.
Gradual fuel
combustion
In addition to the mechanisms of immediate regulation of the
fission reaction, certain changes of a longer-term nature
take place in the active zone during longer operation of the
reactor, affecting (mostly reducing) the yield of the reaction.
Above all, it is clear that during fission the number of atoms of
fissile material gradually decreases, the fuel
"burns out". This reduces the
multiplication factor and in order to maintain the equilibrium
operation of the reaction, the control circuits must gradually
eject the neutron absorbers - the so-called compensating
rods. Another possibility for long-term control and
compensation of fuel combustion is to change the concentration of
a suitable neutron-absorbing substance, such as boron,
dissolved in the refrigerant. This is used in some water-cooled
reactors, where about 1% boric acid is added to the cooling water
and then during operation and fuel combustion, its concentration
is gradually reduced (by dilution with clean water fed to the
primary circuit) to virtually zero before changing the fuel..
When more enriched uranium is used, gadolinium is
sometimes added to the nuclear fuel itself, which serves as a
temporary "combustible neutron poison" (absorbs
neutrons similar to cadmium) - a "burning" neutron
absorber. During operation, it is gradually degraded by
transmutation in the neutron flux (by
neutron absorption it changes to various Gd and Tb isotopes,
which already have a low effective neutron capture cross section), so that as the uranium-235 content decreases, the
gadolinium content also decreases, so that in the fuel cell it
absorbs fewer neutrons in the absorber and more in the fuel -
longer operation time of the enriched fuel with easier
regulation.
Absorption
of neutrons by fission products, neutron "poison" -
"poisoning" of the reactor
Furthermore, during fission, new nuclei are formed, fission
products (detailed above), some of which strongly absorb neutrons
- accumulation of fission products in fuel cells can also reduce
reactivity. If such neutrons absorbing waste products in larger
quantities are formed, they disturb the neutron balance in the
reactor (reduce k) - we say that the so-called poisoning
of the reactor with neutron poison
occurs. Reactor poisoning is quantified by the ratio of the
thermal neutrons absorbed in the fission product
("poison") to the number of neutrons absorbed in the
fuel. Most important thing for reactor poisoning is the nuclide 135Xe,
"xenon poisoning", partly also 149Sm.
During the cleavage of 235U this 135Xe namely is formed directly in only a small amounts
(0.3%), but a larger quantity of about 6% of a fission product 135Te and 135I, whose decay b follows the series
:
135Te (30
sec.) ® 135I (6.7 hours) ® 135Xe (9.2 hours) ® 135Cs (2.6.10 6 years) ® 135Ba (stab.) .
Xenon 135Xe
has an extremely high effective cross sectionfor
neutron absorption 3.5.106 barn (almost perfect neutron absorber!). During normal
operation of the reactor, the occurrence of 135Xe and 135I is in equilibrium, the ongoing fission reaction
constantly produces new 135I, which partially changes to 135Xe and then to 135Cs, but more often changes by neutron absorption to
stable 136Xe
(neutron transmutation combustion 135Xe). 135Cs or 136Xe neutrons almost do not absorb and do not affect the
dynamics of the fission reaction. However, when the reactor power
is significantly reduced or shut down, the equilibrium is
disturbed and 135Xe begins to accumulate in
the reactor, to which the already formed 135I is constantly converted with a half-life of 6.7 hours.
This "xenon poisoning" causes that the reactor to be
unable to start operating again for several hours before 135Xe disintegrates *).
This time, during which it is not possible to restart the
reactor, is sometimes called the "iodine pit"
- after stopping the fission reaction, no more 135I are formed
in the reactor, but this iodine gradually decreases with
decomposition to xenon; the concentration of neutron poison 135Xe first increases
and then gradually decreases by radioactive decay until a state
is reached in which the reactor can be restarted, operated and
regulated.
*) If we still wanted to restart
the reactor in this situation, we would have to eject the amount
of control absorption rods out of the core corresponding to the
absorption capacity of the accumulated xenon 135. If this were
possible at all (for design reasons), it would be very dangerous
- after the start of the chain fission reaction, the neutron flux
would quickly "burn" the inhibiting 135-xenon and the
reaction could start uncontrollably until the crash. It is worth
noting that this very situation of "xenon poisoning"
during the decommissioning of the Chernobyl reactor played an
important disorientation role in the case of
operator errors which ultimately resulted in a fatal reactor
accident, as described below ("Nuclear Reactor Accident").
The degree of
xenon poisoning of a reactor and its time dynamics can vary
widely, depending on a number of factors. It depends on the rate
of time changes in the intensity of the fission reaction (rate of
shutdown or start-up of the reactor), the degree of nuclear fuel
burn-up, the dimensions and the geometric arrangement of the
core. As mentioned above, xenon poisoning is significant
("iodine pit") when the power is suddenly reduced or
the reactor is shut down. When the reactor is shut down slowly, 135I still form and 135Xe "burns"
by neutron transmutation; there is a limit to the shutdown rate
of the reactor at which significant xenon poisoning does not yet
occur and the reactor does not fall into the "iodine
pit". This rate value depends on the fuel supply in the
cells (their degree of combustion) as well as the power of the
reactor from which the change in reactivity is made.
In reactors with a larger core and a
high neutron flux, an inhomogeneous distribution of the neutron
flux can occur - inhomogeneities in reactivity arise. A
random increase in neutron flux density at some point leads to an
increased degradation of 135Xe, thereby reducing neutron absorption at that point
and leading to a further local increase in neutron flux. However,
this will also increase production 135I, it decays into 135Xe, which thus accumulates somewhat locally and reduces
the neutron flux with its increased absorption. This process can
be repeated periodically - so-called xenon
oscillations of the intensity of the fission
reaction occur in various places in the reactor. Under normal
circumstances, such periodic fluctuations in neutron flux in the
core do not significantly affect the overall performance of the
reactor and its stability. However, with a large core volume,
autonomous regions can form, whose xenon inhomogeneities can get
into positive feedback, which can cause an increase in the
amplitude of the xenon oscillations and a violation of the
reactor stability.
Reactor "slagging"
Another nuclide that can affect the neutron balance in the
reactor is 149Sm, which has an effective neutron
absorption cross section of about 4.104 b. It is formed in fission products in the amount of
1.13% as a decay product of the series: 149Nd (2 hours) ® 149Pm (51 hours) ® 149Sm. Due to the smaller effective cross-section and lower
yield compared to xenon, reactor poisoning by samarium is usually
low; since the 149-samarium is stable, it has the character of a
kind of "slag" or "cinders"
which accumulates in the nuclear fuel and prevents its more
perfect combustion.
Even less important are the neutron poisons
157,155Gd
and 155Eu,
which are formed only in very small amounts.
Neutron balance; reactivity reserve
The neutron balance, which is the proportion of
the number of neutrons in successive generations, is therefore
crucial for the proper operation of the reactor. The core of the
reactor must be designed so that the proportion of neutrons in
successive generations is only slightly higher than 1. A certain
potential excess of neutrons, called the reactivity
reserve, is needed as the amount of fuel decreases and
neutron absorption gradually increases during operation, mainly
due to the formation of fission products. During reactor
operation, the excess neutrons are compensated by absorbers,
which are gradually take out from the reactor. The stable and
safe operation of the reactor is due to the reliable control
of the balance between the excess neutrons needed for
operation and the uncontrolled excess, which could lead to an
accident.
Replacement of spent
fuel cells
When the concentration of fissile material decreases to such an
extent that the fission reaction would no longer be maintained
even with the absorbent rods sufficiently displaced, such spent
fuel cells must be replaced with new ones.
This is usually after 12-36 months of reactor operation
(depending on the type of reactor and fuel enrichment); this
period is called the "reactor campaign". For
most types, the reactor must be shut down for this change ("campaign
exchange"), but some types allow for a continuous
stepwise exchange of fuel during operation. Replacing fuel cells
is a very demanding job. In contrast to new (fresh, unused) fuel
cells, whose activity is relatively low (long half-life of a-decay of uranium),
spent fuel cells are highly radioactive (they
contain radionuclides with significantly shorter half-lives; the
activity is inversely proportional to T1/2, as derived in §1.2 "Radioactivity") and no
one is allowed to approach them! The cells are pulled out of the
reactor core by means of remotely controlled manipulators
and are immediately inserted into strong shielding containers.
The radioactive decay of fission products in spent fuel cells is
initially so intense that heat is released and the material is heated
- freshly spent fuel cells must be cooled. The
most common way of their initial storage is placement in a water
pool near the reactor; water provides not only cooling, but also
relatively effective shielding from radiation. Another way is
"dry" cooling, where the fuel cells are placed in
special containers filled with helium, the containers are cooled
by air from the outside. After about 5 years, when the activity
of the material decreases sufficiently, the fuel cells are placed
in intermediate storage and only after many
years they are stored in the final central repository
(unless, of course, their appropriate
further processing is taken, see below).
The problem of cooling
nuclear reactors
Nuclear energy in a fission reactor is released in the form of
kinetic energy of nuclei-fragments and neutrons, which are
converted into heat by their braking in matter.
With the chain fission reaction triggered, it goes without saying
that this large amount of heat must be dissipated by the cooling
medium for further energy use (described
below). However, even after stopping
the chain fission reaction - "shutting down"
the reactor - the heat output does not immediately drop to zero,
but residual heat continues to develop in the
reactor. The fuel cells heat up for some time to
come: they contain a large amount of highly radioactive
fission products, whose intense radioactive
transformations heat these partially or completely spent fuel
cells with their energy *). It is therefore necessary to cool
- aftercool the reactor and fuel cells even after the
reactor has been shut down, otherwise they may be thermally
damaged (even melted). Cooling failure can thus cause
serious and difficult-to-repair damage, in extreme cases even an
accident and destruction of the reactor! - as happened at the
Fukushima nuclear power plant (see "Accidents at the Fukushima nuclear power plant"). The
residual heat output is highest immediately after stopping the
reactor; decreases significantly with increasing downtime **).
*) The residual heat output of a
nuclear reactor depends on the degree of combustion
fuel cells. If we shut down the reactor soon after it is started
with new (fresh) fuel cells, the residual heat output will be
small. However, with the increasing degree of fuel cell burnout,
the concentration of highly radioactive fission products
increases (initially it rises rapidly, but later more slowly: the
most active fission products with a short half-life just
disintegrate - saturation is reached). Thus, when shutting down a
reactor with an advanced degree of combustion, the residual heat
output will be relatively large (it may initially reach up to 10%
of the nominal fission power).
**) After the reactor is shut down, the fission
products in the fuel cells decay radioactively with different
half-lives - the activity and the residual heat output produced
by it decreases (multi)exponentially with time. Immediately after
shutdown of the reactor, the largest radioactivity in fresh
decomposition products in fuel cells consists of short-lived
radionuclides, which decompose rapidly, so that the heat output
initially decreases rapidly exponentially (one hour after
shutdown, residual heat output is less than 2% of operating
fission power). As short-lived radionuclides "dying
out" in fission products, the rate of decrease in residual
heat output slows down constantly - in the first few days it
decreases with a half-life of about 4 days, after a week with
T1/2 about 8 days (radioiodine 131I). After a long time, long-lived radionuclides with
half-lives of hundreds of days to several years survive in fuel
cells in fission products, so that the heat output is already
low, but decreases only very slowly.
Temperature
coefficient of reactivity, cavity effect in the reactor
At low temperatures of the order of hundreds to thousands of °C,
the physical process of fission of heavy nuclei does not depend
on temperature. However, in such a complex mechanical and
hydrodynamic system as a nuclear reactor, temperature changes are
necessarily reflected in geometric proportions, neutron
absorption and moderation, as a result of which the rate of
fission reaction - reactivity, energy or thermal
performance - may depend on temperature. The
dependence of energy output on temperature (with unchanged other
parameters) is expressed by the temperature coefficient
of reactivity, which can be either negative
(as the temperature increases the rate of fission reaction
decreases) or positive (as the temperature
increases, the intensity of the chain fission reaction also
increases).
In water-cooled
reactors, the so-called cavity effect is closely
related to the temperature coefficient of reactivity: the
influence of the amount of water and steam (steam bubbles form
"cavities" in the cooling or moderation system) in the
active circuit on the reactivity. When the temperature rises,
part of the water turns into steam - a "cavity" is
created with a positive or negative effect on reactivity. It is
quantified by the cavity coefficient (void
coefficient), which is a number indicating how much (in what
proportion) the energy output of the reactor will decrease or
increase as the volume of water and steam in the reactor core
changes.
In water-moderated reactors,
the cavity coefficient and the temperature coefficient of
reactivity are negative: as the temperature
increases, the density of water decreases and thus the proton
density, which reduces the moderation effect and fission
reaction, overheating of the core converts part of the water into
steam, leading to a radical reduction and thus to attenuate the
fission reaction. These reactors are "undermoderated". Graphite-moderated
reactors are "overmoderated" and have a positive
reactivity temperature coefficient and cavity coefficient: as the
temperature increases and the amount of steam in the reactor core
increases, the amount of water-absorbed neutrons decreases, so
the number of slow neutrons capable of further fissioning uranium
increases (the main neutron moderator is not water but graphite,
the amount of which in the reactor core is independent of
temperature; water somewhat absorbs neutrons). From a control and
safety point of view, reactors with a negative temperature
reactivity are more advantageous, while reactors with a positive
temperature reactivity are more difficult to regulate cooling and
energy output, with a higher risk of unstability, faults and even
the possibility of accidents.
Various
constructions and functioning of nuclear reactors
The first nuclear reactor for the production of electricity was
launched in 1954 in Obninsk in the USSR. Since then, the design
of the reactors has undergone a number of changes and technical
improvements. There are currently a number of types of nuclear
reactors in operation in different countries, and some new
technical solutions are being developed. We will briefly mention
here only a few of the most important types of nuclear reactors.
Types of nuclear reactors can be classified according to several
basic aspects :
Division of
nuclear reactors according to the purpose of use
According to the purpose of use,
nuclear reactors can be divided into three groups :
- Research and experimental reactors are
usually smaller instruments used for experiments in various
fields of physics, engineering (such as materials engineering,
metallurgy), biology, medicine. The intense flux of neutron
radiation can be used for neutron activation analysis
(§3.4, section "Neutron
activation analysis"), for neutron
radiotherapy (§3.6, section "Neutron
capture therapy") and some other
applications (see also the section "Irradiation in
nuclear reactors" below). This includes also school
reactors for teaching and prototype reactors for
verification of new concepts and design solutions.
- Transmutation reactors for the
production of radionuclides (see §1.4, section "Production of
artificial radionuclides"), including
some transurans, especially plutonium-239 (which can unfortunately be misused for military
purposes...).
- Power reactors, used to produce heat
and electricity, are the most important and numerous group of
nuclear reactors. They are mostly large and expensive
constructions, but there are also several smaller mobile
reactors working on board submarines and large icebreakers.
Below we will focus mainly on power reactors.
Development
generations of nuclear reactors
In terms of gradual technological
development, nuclear reactors are divided into 4 or 5
generations. Generation I reactors were built in
the 1950s and 1960s. The experience with these
"prototype" reactors was followed by the second
generation of reactors (so far the most frequently
used), mostly light water pressure or boiling, or heavy water,
graphite, water moderated - or carbon dioxide cooled. This is
followed by Generation III reactors with a
number of technical improvements, especially in terms of safety,
reliability and economy of operation, with a higher degree of
fuel burn-up and a smaller volume of radioactive waste. Generation
IV reactors although they build on previous generations,
they are based on completely new technologies and operating
principles; they are mostly still in the stage of experiments and
projects. These reactors should allow the use of all
potential nuclear fuel - not only conventional fissile
uranium-235, but also uranium-238 and thorium-232. It also uses fast
neutrons (see "Fast FBR Propagation Reactors" below) and nuclear
transmutation technologies, which would make it possible
to "burn" even previously unusable fuel (235U in low
concentrations, mentioned 238U and 232Th), as well as the resulting transurans. This
would lead not only to an increase in the efficiency of the use
of natural resources and energy recovery, but also to a reduction
in the volume, activity and hazard of nuclear waste. The most
advanced technology of this kind, which could perhaps already be
included in Generation V, is the accelerator-controlled
transmutation technology ADTT, described below.
In the following, we will briefly describe some
significant designs of nuclear reactors (partly "across
generations") :
Graphite-moderated
water-cooled reactors
The first types of reactors were single-circuit,
the moderator was graphite and the coolant was
water, the steam of which is led directly to the turbine. Such
designs were, for example, RBMK ("high
power, channel") reactors used, inter alia, at the Chernobyl
nuclear power plant. In the graphite block, cooling channels
(tubes) pass vertically, in which uranium-enriched fuel cells are
located. There are also channels for inserting and removing
control rods. Water flows through the cooling channels from the
bottom up, which is heated by the released energy, removes heat
from the reactor and in the upper part it changes into steam, led
to the turbine el. generator. The cooled steam and condensed
water are then returned to the bottom of the reactor.
Graphite termination of boron
control rods
In some graphite-moderated reactors (RBMK), absorption-control
rods were provided at their ends with a graphite part
(boron at the top, water gap in the middle,
graphite at the bottom) for more
homogeneous neutron flux and smoother positive or negative
control during ejection and inserting the rods. The graphite part
caused "displacement" of water from the channel as the
rod moved. This was supposed to lead to a wider range of
neutron flux regulation, as the insertion of the
graphite part (slightly moderating) slightly increased the reactivity compared to
"empty" channels with water (which
easily absorbs neutrons). It should also
have slightly increased the use of lower concentrations 235U in fuel cells. In
practice, however, this "trick" did not apply too much,
the effect is not significant and, according to some opinions, it
could cause instability in the control of anomalous situations (though not proven, is briefly mentioned below in the
passage of "nuclear reactor accident at Chernobyl").
Reactors of this type they had, in
addition to a simpler design, three advantages :
1. Since the graphite moderator absorbs neutrons
only slightly, less uranium enrichment in the
fuel cells was sufficient (around 2%).
2. Easy power control and the possibility of
shutting down only part of the reactor.
3. The division of the fuel cells into
independent channels allowed the gradual replacement of the fuel
cells during operation, without a complete
shutdown of the reactor. The fuel could be exchanged gradually by
individual channels, while the rest of the reactor worked
normally (it was also easier to obtain the
resulting radionuclides, including plutonium 239, from the
exchanged spent rods).
However, these reactors have also
been shown to have disadvantages (which ultimately predominate) :
a) The single-circuit design
can lead to radioactive contamination of the turbine and an
overall greater risk of radioactivity leakage.
b) Positive temperature coefficient reactivity:
as the temperature increases and the amount of steam in the
reactor channels increases, the amount of water-absorbed neutrons
decreases, so that the number of slow neutrons capable of further
fissioning uranium increases. The main moderator of neutrons is
graphite, the amount of which in the core of the reactor is
fixed. Elimination of this phenomenon places increased demands on
control technology.
c) High demands on the tightness
of a large number of channels.
A positive temperature coefficient
of power leads to the risk that in the event of a leak or boiling
of water, the fission reaction continues at an increased rate
(moderating effects of graphite persist) and if the absorption
rod control is not applied, the core may overheat
until the reactor crashes. These circumstances,
combined with serious operator errors, became
fatal for the RMBK-1000 reactor of unit 4 of the Chernobyl
nuclear power plant (described in the
passage "Chernobyl nuclear reactor
accident"). Otherwise, a number of reactors of this type, with
proper operation, worked for several decades, reliably
and safely...
Water
moderated reactors
PWR - pressure water moderated
Reactors type PWR ( Presurized Water
cooled and moderated Reactor), also known as the
WWER (Water- Water
Energy Reaktor) are now the
most common type of reactors. The moderator and coolant
are ordinary water, so it is also referred to as
"light water". Reactor cooling is two-circuit:
in the primary circuit, water flows under high pressure at a
temperature of about 300 °C, in the steam generator it heats the
water of the secondary circuit and only the steam generated here
drives the turbine el. generator. These reactors are
characterized by high operational safety and
accident resistance. The use of water as a coolant and moderator
leads to a negative temperature coefficient of reactivity
: 1. As the temperature increases, the density
of water decreases and thus the proton density, which reduces the
moderating effect and the intensity of the fission reaction. 2.
Overheating of the core and conversion of water into steam would
lead to a radical reduction (or disappearance) of the moderating
effect and thus to a attenuation of the fission reaction.
The two-circuit solution also virtually
eliminates the possibility of tritium contamination. The primary
circuit is not in direct contact with the turbine, but water or
steam from the reactor transfers heat indirectly to the
non-radioactive water that drives the turbines. The reactors used
in our country (Jaslovské Bohunice,
Dukovany, Temelín) are of the WWER type.
BWR - Boiling Water Reactor
The second most common type of reactor is BWR
(Boiling Water Reactor). The water, which serves as both a
coolant and a moderator, is heated to boiling directly in the
pressure vessel of the core and this steam directly drives the
turbine - the BWR reactors are single-circuit.
Gas-Cooled
Graphite Moderated Reactors
GCR - Gas- Cooled Graphite Moderated Reactor
In a GCR (Gas Cooled & Graphite
Moderated Reactor) reactor, the core consists of graphite
moderator blocks through which a large number of fuel rod
channels pass (can be replaced during operation). The refrigerant
driven by the core is carbon dioxide gas, which
after heating is fed to a steam generator, where it heats the
water of the secondary circuit and the generated steam drives the
turbine.
HTGR - high temperature gas cooled graphite moderated
reactor ("pebble" reactor)
The HTGR (High Temperature Gas Cooled Reactor) reactor differs
from other types of reactors in the arrangement of the fuel and
the core. The fuel is highly enriched uranium in the form of
uranium dioxide, whose small spheres (0.5 mm)
are dispersed in large numbers in spheres of graphite about 7 cm
in diameter - some pebbles, or in hexagonal
blocks. Fuel balls or blocks are loosely "strewed" or
stacked in the active zone, the spent ones are gradually removed
from the bottom and freshly filled from above. The refrigerant
driven by the core is helium gas, which after
heating is led to the steam generator, where it heats the water
of the secondary circuit and the generated steam drives the
turbine. The advantages of reactors of this type are smaller
dimensions, relative simplicity and less economic demands - they
resemble a bit of "permanent heating stoves", into
which coke is poured from above and ash and slag are removed from
below. They can therefore be a promising solution..?..
Heavy water
moderated reactors
As mentioned above, deuterium in the form of heavy
water (D2O) has very good moderating properties, which allows the
use of natural or only weakly enriched
uranium as fissile material; it is mostly used in the
form of oxide (UO2). Several types of "heavy water" reactors
have been developed in which heavy water is moderator, but the
individual variants differ in refrigerant and heat transfer
method :
PHWR (Presurized Heavy Water Moderated and Cooled Reactor) -
a pressure reactor moderated and cooled by heavy water
uses natural uranium as fuel, the refrigerant and moderator is heavy
water, which transfers its heat from the primary cooling
circuit to ordinary water in a steam generator, from where the
steam drives the turbine;
HWLWR (Heavy Water Moderated Boling Light Water Cooled
Reactor) - heavy water moderated and light water cooled boiling
reactor;
BHWR (Boiling Heavy Water Cooled and Moderated Reactor) - boiling
reactor moderated and cooled by heavy water ;
HWGCR (Heavy Water Moderated Gas Cooled Reactor) - a heavy
water moderated and gas cooled reactor .
The technical details of the construction of various
types of nuclear reactors are already outside the scope of our
physically focused treatise...
Molten
salt-based nuclear reactors ,
also called "salt reactors" and are
abbreviated as MSR (Molten
Salt Reactor), are still in the experimental and design
stage. This is an interesting and promising
solution for Generation IV reactors using molten fluoride
salts as solvents for nuclear fuel, as well as for
cooling and heat dissipation. Fuel in the form of uranium
fluoride UF4, plutonium fluoride PuF3, or thorium fluoride ThF4, can be dissolved in salts (fluorides) of lithium LiF
*), beryllium BeF2, or sodium fluoride NaF. Thus, nuclear fuel is not
fixed in the solid structure of fuel cells, but circulates
dissolved in a liquid state.
*) It is not possible to use natural
lithium, where 6Li strongly absorbs neutrons, but the almost pure (or
highly enriched) isotope 7Li, in order to avoid the loss of thermal neutrons,
which are the main "engine" of nuclear fission.
The reactor can work both in the classical
variant with neutron moderation (using graphite) for uranium
combustion or thorium transmutation, and without moderation as a
fast reactor for plutonium combustion. The primary circuit may
also contain a chemical-isotope separation unit
for the continuous removal of fission products or for the
isolation of fuel in a breeding reactor. The most advanced
reactors of this type would be the ADTT transmutation reactors described below.
Molten fluoride or chloride salts
can also be used for cooling and heat dissipation for further
energy use. The use of molten salts as a coolant has the
advantage of a high volume heat capacity, which makes it
possible to remove heat from the reactor core
very efficiently. Reactors of this type operate
at a high temperature (around 1000 °C), so the
heat generated can be used not only for the production of
electricity, but also for the efficient production of
hydrogen as an important technological and energetic
gas.
Compact self-regulating reactors
In the basic description of the principles of nuclear reactors,
it was mentioned that an effective control mechanism of the
fission reaction rate can be to change the moderator
concentration in the reactor core (see
section " 3. Reactor control by controlled
moderation" above). By a
suitable technical construction and material design of the
moderating substance, a negative reactivity
temperature coefficient can be achieved, which can
be used for the autoregulatory operation of the
reactor on the basis of the operating temperature.
Based on this principle, compact self-regulating reactors
small dimensions are very interesting at the project stage, which
do not contain any mechanically moving parts inside the core, are
maintenance-free for the life of the fuel and are also safe
against accidents thanks to the negative reactivity temperature
coefficient.
Hydrogen-moderated small nuclear reactor
At advanced stage of development is the project of a small
nuclear reactor, whose fuel is uranium (enriched
to about 5% 235U) in the form of uranium hydride UH3, placed in the shape
of granules in a gaseous hydrogen atmosphere. The
hydrogen contained in UH3 hydride serves as a neutron moderator.
Autoregulation is achieved by a thermally induced balance between
chemical formation and decomposition of the UH3 moderator in a
gaseous hydrogen atmosphere: UH3 « (800 °C) « U + 3H. As the
temperature rises (to about 800 °C), UH3 decomposes and the reaction slows down due to the lack
of moderator; on the contrary, as the temperature decreases,
hydrogen binds to uranium in a hydrogen atmosphere, the
concentration of the moderator UH3 increases and the fission reaction accelerates. The
assembled reactor is started by introducing hydrogen gas into the
core, by discharging the hydrogen the reaction can be stopped at
any time. Also, each time the core overheats, the reaction stops.
Molten potassium is planned as a refrigerant.
Fast breeder
reactors and technology ADTT
Some other new and promising types of nuclear reactors (fast
breeder reactors FBR and technologies designed reactors ADTT ) mentioned below.
Irradiation
in nuclear reactors
In addition to energy use, the nuclear reactor can also be used
as a powerful source of neutrons for irradiation
of various materials in which nuclear reactions (n, g), (n, p), (n, d),
(n, a)
etc., arises artificial radioactivity. For this
purpose, irradiation chambers or channels are
located in the active zone of the reactors, where the irradiated
material is inserted. Many radionuclides, including some
transurans (especially plutonium), can also be separated from the
spent nuclear reactor fuel. The production of
radionuclides is discussed in §1.4.
"Radionuclides", part "Production of artificial
radionuclides", in §3.4
is described Neutron activation analysis.
Natural nuclear
reactors ?
As experience shows, many physical phenomena that we study in
laboratory conditions also occur in nature. The successful
management of the fission reaction in reactors raises the
question whether the chain fission reaction cannot occur even in
nature?
The basic condition for the chain
fission reaction is that 235U (the only naturally occurring isotope that is
potentially able to sustain the chain fission reaction) be
present in a sufficient concentration in an
appropriate volume greater than the average path length of the
fission neutrons. Today's concentration of 235U is too low (approx. 0.72%) and far from sufficient for
a chain reaction even in the richest uranium deposits. However, 235U decays about 6
times faster than 238U, so that in the distant past the proportion of 235U may have been significantly higher. As early as 1956,
the Japanese nuclear physicist P.Kurota pointed out the
possibility of a chain fission reaction in natural uranium
deposits.
About 2 billion years ago, 235U was about 3%
(similar to enriched uranium used as fuel in today's nuclear
reactors), which under favorable conditions is already sufficient
to trigger a chain fission reaction. This "favorable
condition" *) is the presence of a neutron moderator
- a substance that slows down the neutrons emitted during fission
to better cause the fission of other nuclei. Under natural
conditions, such a moderating substance could be underground
water seeping through pores in a uranium deposit.
*) On the contrary, an
unfavorable circumstance could be the presence of larger amounts
of boron, lithium, cadmium and other elements that effectively
absorb neutrons and thus stop the fission reaction. However,
these substances are not commonly found in larger quantities in
uranium deposits.
In 1972, during the analysis of
uranium ore samples from the Oklo opencast mine
in Gabon (in western Equatorial Africa), it was found that the
ore from some parts of this deposit is depleted
of uranium 235U. Further analyzes showed an increased occurrence of
some lighter elements, especially xenon, which
often arise during the decay of the heavy core 235U in two parts.
These circumstances suggest that in the distant past there was a spontaneous
ignition of the chain fission reaction, in which part of
the 235U
was consumed ("burned out"), split into lighter
elements. As there are no signs of rock melting or explosion, it
was a "controlled" natural reactor: as
the temperature rose due to the energy released by the fission,
some of the water evaporated and the loss of this moderator
stopped the reaction. After cooling and soaking in the water
again, the fission reaction could start again. In this way, the
nuclear fission reaction could be ignited cyclically for many
thousands of years.
Note: Such
"moderation-controlled" nuclear reactors can also be
used in current technical practice, as described above in the
section "Compact self-regulatory
reactors".
Such places increased concentrations
of uranium could be in the crust more so in the distant past,
some uranium deposits could act as a natural analogue of
nuclear reactors ! Now, however, after such a
long time, all traces of the activity of these natural nuclear
reactors are almost wiped out: fission
radioactive products have long since disintegrated, other
daughter elements have been washed away by groundwater, and the
integrity of deposits has been violated during geological
processes. Some ancient natural reactors could
perhaps might reveal an increased
incidence of xenon in the gas escaping from underground
..?..
In even
earlier times - during the formation of planets and the entire
solar system after the explosion of the parent supernova - there
were probably often places with an increased concentration of 235U or other fissile
materials. At that time, there were probably a number of massive
flares of chain fission reactions, often of large scale and
explosive nature.
Otherwise,
more than 12 billion years ago, supernova explosions
was often occurred in the universe (and continue
to do so today), that eject clouds of hot matter enriched with
heavy elements, including uranium and transuranics (see, for
example, "Cosmic
Alchemy"). Shock
waves in such clouds can form densities with the conditions for
triggering a chain fission reaction, but compared to the massive
flow of energy from a supernova explosion, the energy released
during the chain fission reaction is of no greater astrophysical
significance.
Other
fissile materials. Transmutation. Breeder reactors.
So far, we have shown the fission of heavy atomic nuclei and
their use in a nuclear reactor on the most common example of uranium
235U, which is the only fissile material
available in nature (artificial 233U also appears to
have similar properties). However, there
are other heavy nuclei capable of fission reactions under the
influence of neutrons. The most common isotope of uranium 238U
(representing 99.3% of natural uranium) can only be cleaved by
fast neutrons with a kinetic energy higher than 1.2 MeV and is
not suitable for direct use in a chain fission reaction. A
similar situation is with thorium 232Th. However, there are ways in which
uranium 238 and thorium 232 to rework for fission and obtaining
nuclear energy :
Transmutation
fuel chains ,
which allow by nuclear reactions with neutrons to convert
(transmute) the nuclides of uranium-238 and thorium-232
into elements, capable of chain cleavage reaction (plutonium-239 or uranium-233). We
will first introduce the method of "breeder" reactors,
which is already being used now, the second way of the future
(accelerator-controlled transmutation technology) will be
mentioned below.
Fast breeding reactors with uranium-plutonium fuel cycle
Irradiation of uranium nuclei 238U with neutrons leads to the reaction :
238 U 92 (n, g) 239 U 92 ® (b - ; 25min
) ® 239 Np 93 ® (b
- ; 2,3 days ) ® 239 Pu 94 ,
which forms an important transuranic element plutonium
239Pu *), which, like 235U, cleaves by fast and slow neutrons and undergoes a
nuclear chain reaction even at a much smaller critical mass
(about 10 kg) than uranium; it can therefore be used in a nuclear
reactor.
*) Plutonium 239 is a very dangerous
radionuclide: it is a-radioactive with a
half-life of 2.44.104 years, easily contaminates, has high radiotoxicity (and
chemical toxicity) and with a larger amount there is a high risk
of radiation accident. For its low critical level is abused,
inter alia as a primer to thermonuclear nuclear weapons....
This way using 238U is used in reactors with a fast
(non-slow) neutrons *) having no moderator, but
contains more fissile material (239Pu, 235U) in the form of more enriched uranium 238 to about
20-50% (effective cross section of
plutonium and uranium for the fission reaction with fast neutrons
is lower than for slow neutrons). A higher
concentration of fissile material leads to a more intense release
of heat in the core per unit volume. Thus, a fast reactor must
have more efficient heat dissipation using a medium capable of
operating at a higher temperature, about 500 °C. Therefore,
water (which would, after all, slow down
neutrons) is not used for cooling in the
primary circuit, but, for example, molten sodium metal,
which has a much better thermal conductivity and a much higher
boiling point (almost 900 °C) than water.
*) When using slow neutrons, the cleavage
of 239Pu
nuclei occurs in only about 65% of cases. In other cases, the
plutonium nucleus 239Pu absorbs the neutron to form the isotope 240Pu, which does not
have fission properties. However, if the plutonium-239 nucleus is
affected fast neutron (instead of slow), in the
vast majority of cases it will not be absorbed, but the 239Pu nucleus will
split, producing 2-3 more neutrons for the chain fission reaction
and for the production of more Pu-239 (from
uranium-238) than the reactor consumes.
The cleavage of 239Pu by fast neutrons
produces an average of 3.02 new neutrons /1 fission. Less than
two neutrons are consumed on average for further fission, and the
remainder, a little more than one neutron on average, is captured
by 238U
nuclei, producing 239U, which is converted to plutonium 239Pu by the two beta conversions mentioned above. Thus, plutonium
is constantly formed from uranium 238U here; to increase
the yield of plutonium, the active zone of the enriched fissile
material is surrounded by an additional layer of 238U (unenriched).
Reactors of this type are sometimes called Fast Breeder
Reactors (FBR) because they use fast
neutrons to "multiplies" the fissile material plutonium
in them, which is produced slightly more than is consumed for
fission.
Through plutonium, it is possible in
principle to recover more than 90% of uranium-238 (and thus
natural uranium) and thus multiply the available natural sources
of fissile material for nuclear energy.
Breeder reactors with
thorium-uranium fuel cycle
In a similar way, the possibility of using thorium 232
Th is considered, which would change by neutron capture
to 233Th
and this would b -decay gradually (over protactinium 233 Pa) to transmute uranium
233U :
232 Th 90 (n, g ) 233 Th 90 ® (b - ; 12min
) ® 233 Pa 91 ® (b
- ; 27 days ) ® 233 U 92 .
Uranium-233 is as good a fissile material, capable of chain
fission reaction, as 235U or plutonium-239. "Excess" neutrons during
fission 233U
then transmutes thorium-232 to uranium-233, so that the 233U fissile material
"multiplies" here at the expense of thorium *). This
process can take place with both fast and slow neutrons (unlike
the above-mentioned uranium-plutonium reactors operating only
with fast neutrons).
*) The problem here is the somewhat long half-life of the
intermediate protactinium-233 to fissile uranium 233U - 27 days. During
this time, 233Pa can be converted to protactinium-234 by further
neutron capture due to the intense neutron flux, which is
converted to uranium-234 by b-
radioactivity (with a half-life of about 7
hours); it cannot be used as fissile fuel. It would therefore be
advantageous to continuously withdraw
protactinium-233 from the reactor, leaving it in a suitable
container (outside the reactor) decay into uranium-233 and back
into the reactor insert to the thus formed "finished" 233U. It may be
possible to implement in reactors with fuel dissolved in molten
fluoride salts with a continuous chemical isotope
separation.
Thorium-uranium reactors could be
promising given that thorium reserves in the Earth's rocks are
about 4 times larger than uranium. A certain
advantage of this reaction is that it generates radioactive waste
with a lower specific activity (standardized on the energy yield of the reaction) than in the uranium-plutonium cycle *).
*) Especially heavy long-lived
radionuclides such as neptunium, americium, curium are formed
here only in negligible amounts, because the nucleon number of 232Th is 6 units lower
than 238U.
In every activity - in industry, transport, agriculture, health,
science and technology, laboratory work, as well as in everyday
life, sometimes something fails, goes wrong, "breaks" -
an accident occurs.
Nuclear reactor
accidents
A serious malfunction of a nuclear reactor, associated with its
irreversible damage, is referred to as an accident.
Such an event can in principle occur either by a technical
failure or by a human factor, or a
combination of both (incorrect procedure of
workers in solving a technical failure).
Possibly also due to natural events (Fukushima).
From the point of view of operation, such
a reactor accident can occur in two phases :
× Accident in the phase of an ongoing chain
fission reaction
it can be caused either by
an uncontrolled running of the chain reaction (failure of the
neutron flux control) or by a failure of the cooling and drainage
of the thermal power of the reaction. This is usually a very
severe accident, associated often with the melting of the inner
part and the destruction of the reactor.
× An
accident in the shut down phase of a reactor may
be caused by insufficient cooling of the residual heat output ("Nuclear reactor cooling problem"
mentioned above), as well as some
manipulations during fuel cell replacement.
In principle, a nuclear
reactor can not explode nuclear like an
"atomic bomb" (the physical
conditions mentioned above in the section "Uncontrolled
chain reaction - nuclear explosion"
are not met here).
However, the increased temperature may cause a rise of pressure,
which can lead to pressure explosion. In the
enormous increase in temperature can cause decomposition of water
into hydrogen and oxygen and subsequent chemical
explosion. If the temperature of the fuel cell exceeds a
value of about 600-1000 °C, their hermetic shell (zirconium alloy) is thermally
damaged and the radionuclides contained, especially
fission products inside, may leak into the surrounding
environment, so in the event of a more serious accident of a
nuclear reactor, radioactivity *) may also leak
into the surrounding space and the environment. The technical
accident of the reactor can thus be accompanied by a radiation
accident (in the terminology of
radiation hygiene - §5.6, part "Radiation
accidents") and radioactive contamination. The
accident at the Chernobyl nuclear reactor and the recent accident
at the Fukushima nuclear power plant are briefly described below.
Some other aspects are discussed in the section "Safety
and risks of nuclear energy").
*) In the event of a nuclear reactor
accident with a leak of radioactivity, there is mainly a leakage
of fission products. When overheated, substances with a low
boiling point are preferably emitted into the air and evaporate
easily; it is mainly radioiodine 131I and cesium 137Cs. Fortunately, heavy radionuclides, such as uranium or
high-boiling plutonium, are released very little. However, if the
tightness of the fuel cells is broken, all radionuclides can leak
into the water (primary, nuclides formed by nuclear reactions and
fission products, to the extent dependent on their amount and
solubility).
We will briefly mention the two most
serious cases of nuclear reactor accidents (focusing
mainly on proven physical and technical aspects,
without speculation about the subjectively debatable social
background) :
Chernobyl
nuclear reactor accident
Serious operator errors,
partly in combination with the positive temperature coefficient
of graphite-moderated reactors, have become fatal for the
RMBK-1000 reactor of Unit 4 of the Chernobyl nuclear power plant.
On April 26, 1986, technicians conducted a "safety"
experiment with a partial shutdown of the reactor to verify what
power for the water pumps of the primary reactor unit could still
be obtained from the inertial deceleration of the unit
turbines (bridging about 1 min. - in case
of power failure) after shutdown. Due to
broken coordination (interruption of the
test for several hours - economic requirement for increased
electricity supply to "meet the plan" - caused this
very non-standard operation to be completed by another group of
workers, little familiar with the test procedure) reactor power dropped to too low and operators then
failed to "revive" the reaction; in fact, a larger
amount of 135Xe has accumulated in the fuel cells,
which absorbs neutrons very efficiently - the so - called "xenon
poisoning" mentioned above in the passage
"Neutron absorption by fission products,
neutron" poison "-" reactor " poisoning". In this situation, the only sensible solution is
to shut down the reactor, wait a few hours and
then start it in the standard way.
The technicians did not recognize
it, their profession was mostly electrical engineers who did not
know the complex details of the dynamics of the nuclear reactor.
Above all, however, they were under pressure from an ambitious
and despotic boss, who eagerly tried to complete the test (for which he would have a functional promotion). In an effort reactor at all costs fast
"start" committed a fatal error : off
emergency protection of the reactor and pulled out
from the core of almost all control rods above the permissible
limits (with graphite end) - so high, the control rod in a functioning reactor with
nuclear fuel must never extend !
As a result, the fission reaction
eventually started, but it was unstable way,
with disconnected control and emergency protection! Another
mistake was to disconnect the turbine and some circulation pumps
of the primary circuit, straightforward in the spirit of the
ongoing test (which in this situation was
already wrong and pointless, he had to stop -
but the authoritarian boss insisted on it, had a personal career
interest...).
The flow of coolant through the
reactor began to decrease, the temperature rose, and excessive
steam generation and accumulation occurred in the core. In this
unacceptable state, a positive temperature coefficient
("cavity effect") of the graphite-moderated reactor manifested itself -
an increase in steam led to an increase in the fission reaction.
And most importantly, due to the increase in neutrons, almost all
135Xe transformed very quickly to stable 136Xe, which no longer
absorbs neutrons. As a result, the only neutron absorber that was
in the core at that time disappeared, as the
absorber rods were ejected! This led to a sharp uncontrolled increase
in the chain fission reaction. Emergency insertion of
the boron absorption rods failed, the reactor was enormously
overheated and the channels were deformed *),
the control rods jammed at about one third of the height. The
chain fission reaction started uncontrollably at
many times the nominal power, which resulted in overheating and
reactor failure.
*) According to some opinions, the graphite
ends (tips) of the control rods (mentioned above in the
passage "Controlled chain reaction",
paragraph 2. Neutron absorption - "Graphite-boron
combination?") also contributed to the accident .
However, this was of only marginal importance in
terms of the cause, the perpetrators of the accident only excused
themselves. During the emergency insertion of control rods, a
slight short-term increase in reactivity was reflected only in a
certain part. The real cause of the accident was the late
insertion of the control rods (which were previously unacceptably
high ejected) into the already overheated
reactor with thermally deformed channels. If the
emergency insertion of the control rods were started only a few
minutes earlier, no accident would occur and the reactor could in
principle be restarted in a regular manner after about 1 day
(although after such a serious emergency it would certainly be
appropriate to perform a thorough technical revision reactor! -
which, moreover, so in the next few days were planning) ...
Steam pressure
caused expolosive tearing the lid of the reactor,
the reactor entered air, graphite moderator blocks began to burn.
There was also an explosion of hydrogen and oxygen, caused by the
decomposition of water in contact with the hot materials of the
reactor. The inner part of the reactor core has melted,
the reactor was destroyed. The fire of the reactor unit lasted a
whole week, dark smoke rising from the building, containing a
large amount of radioactive isotopes.
Atmospheric currents - the wind that was westward at the time,
carried a radioactive cloud up to several
hundred kilometers above Europe. With rainfall, there was a radioactive
contamination of the natural environment - water, soil,
plants.
The main
contaminants were radioiodine 131 I and cesium
137
Cs. Iodine 131I gradually
disintegrated over several weeks with a half-life of 8 days, 137Cs (half-life 30 years) has been
measurable for many years. With further rains, which are no
longer radioactive, contaminating radionuclides gradually entered
deeper soil layers and biosphere contamination was minimal.
E.g. at our nuclear medicine workplace in
Ostrava-Poruba, where it rained during storms on May 1, we
observed an almost twofold increase in the value of the natural
radiation background on radiometric instruments in the following
days. In rainwater samples, we measured the activity of about 2-5
kBq/liter, especially radioiodine I131, on well scintillation spectrometers. I also took
samples of rainwater in Konice in the Drahan Highlands and in
Holešov in the Kromeríž region, where I had a trip in
those days. In Konica there was also increased radioactivity
(about half as less as in Ostrava), in Holešov, where it did
not rain in those days, no increased radioactivity was measured
in the samples above the usual natural background. Communities
nuclear medicine professionals produced recommendations that in
areas with increased contamination should be administered,
especially for children, a small amount of iodine
(non-radioactive) to displace the accumulation of radioiodine in
the thyroid ..?..
When destruction of the
nuclear reactor at Chernobyl was extensive contamination
of the environment with radioactive fission products and exposure
of 232 people with high doses of radiation
(units up to tens of Sv), associated with deterministic effects
and acute damage to health; in 31 cases there were even lethal
effects. Of these, 2 workers were killed directly when the
reactor exploded, but even if this did not happen, they would
receive a lethal dose of radiation! Many hundreds of other people
received radiation doses of tens to hundreds of mSv, for which an
increased incidence of stochastic effects can be expected (by at least 1%, although this has not been directly
confirmed ...).
The burnt-out nuclear
reactor building was removed and a large concrete cell was built
around the remains of the reactor - "sarcophagus"-
to prevent further contamination of the natural environment. It
acts as a kind of temporary "intermediate storage" of
nuclear waste. It is planned that after several decades
(2030-40?) the reactor core will be mined and after appropriate
modifications or reprocessing placed on permanent storage
of nuclear waste. It will be a difficult task due to the high
radioactivity and a large volume of nuclear waste and
contaminated materials. Such a central
repository is planned to build at the nearby campus of Chernobyl.
Among experts there were even proposals to re-build a nuclear
power plant Chernobyl (of course, with new technology),
politicians and the public are against ...
The
Chernobyl accident became certain negative milestone
in nuclear energy and radiation protection; it was a
big mistake that happened! Unfortunately, it is not emphasized
that it was caused by the human factor - the
accident was actually caused by one person who,
in his despotic ambition, arbitrarily violated basic safety
standards and threw the reactor into a completely unacceptable
mode. Such an "barbaric" treatment would not be
tolerated by any existing reactor, in any case it would result in
a serious failure or even an accident. Thus, no
technical error of the reactor (which, if
properly operated, can function reliably for several decades) *) or of the political system (as
ideologically argued by the supporters of the "Cold
War"), and certainly not
the risk of nuclear energy as such (as it
is still misinformed by opponents of the "atom") !
*) Perhaps the only relevant design error
of the RMBK-1000 reactor was the user
possibility of inadmissible ejection of the absorption rods;
it was assumed that the reactor would be operated by
professionally erudite and responsible workers who would not do
such "nonsense"... If this were mechanically blocked
(and complex mechanical work would have to be done in service
mode), the mania of the chief engineer would not pass and no
accident would occur. Many reactors of this type are still
operating reliably, some of which have operations planned for
2030 and beyond ...
This
tragic event has led to an unjustified attenuation of
nuclear energy, radiophobia and
large-scale tightening of safety regulations and
standards of radiation protection not only in nuclear energy, but
in the whole area of applications of ionizing radiation (this resulted in excessive bureaucratization of
radiation protection - cf. the section "Bureaucratic requirements for
radiation protection" in
§5.8). On the other hand, it has become a lesson
to prevent similar events.
During the
modernization of the existing RBMK reactors, some technical
modifications and revisions of their operating modes were
performed. For example, the insertion speed of the control rods
was increased, their construction was modified (proportions
between the boron part and the graphite ends; these are just
unimportant details). Most importantly, however, a technical
barrier was implemented against arbitrary inadmissible
pulling out of control rods, which was the main cause of the
accident.
An accident as large as in Chernobyl will probably never
happen again !
The
accident at the Fukushima nuclear power plant
The last accident at the Fukushima
nuclear power plant had completely different causes (and fortunately a much smaller extent of aftermath) than the Chernobyl accident. This large power plant is
located on the northeast coast of the Japanese island of Honshu,
was built in 1966-79 and is equipped with six boiling reactors
(BWR; BWR-3,4,5) with a total electrical output of 4700 MWe. The
cause of the accident was a huge natural disaster
- a devastating earthquake (9 magnitude) with a
subsequent tsunami that occurred on March 11, 2011. At that time,
there were four reactors in operation, the other two were
regularly maintained.
At the first signs of earthquakes
(recorded by sensors) are all running reactors immediately
automatically shut down, the water pump, powered by
backup diesel generators, regular ensure cooling of the
residual heat in the active zones (diesel
generators were used because the earthquake was interrupted power
supply - "black -out").
So far, everything would be fine.
However, about an hour after the
earthquake, a tsunami, more than 10 meters high,
flooded the coast ! Nobody expected such a high wave in the
construction of a flood barrier on the coast. Pumps and diesel
generators located in the lower parts of the reactor buildings
were flooded with water and stopped working.
This interrupted the cooling of the residual heat, the
temperature rose in the core, the water boiled, the fuel rods
became hot and hydrogen and oxygen were formed in contact with
water and steam. Hydrogen and oxygen exploded, damaging the
reactor structure. Some radioactive fission products began to be
released from the superheated fuel rods. The reactor buildings
also housed tanks with spent fuel cells (not
only from Fukushima, but also from other nuclear power plants), from which radioactive substances also began to be
released. Further overheating was temporarily prevented by
cooling with seawater. After a few weeks, the situation partially
stabilized, but four reactors were severely damaged or destroyed.
A relatively large amount of radioactive substances escaped into
the seawater, which, however, soon dispersed in the large
dilution space and diluted to below-limit specific activity.
No one was to blame
for the Fukushima accident ! To the address of
"nitpickers", who claim the opposite and only criticize
and "look for lice", one can aptly respond with the
proverb " After the battle, everyone is a
general". After all, since the world's mass
media it has been very unfair, as
disproportionate (and often distorted)*) paid attention to the radiation aspects of this
accident, which was in fact just a small episode in the terrible tragedy
that struck Japan and killed thousands of people! When the
Fukushima disaster no one perish, and no one was
irradiated with high radiation dose at which it was threatened
deterministic radiation effects. According to the Japanese Atomic
Energy Agency, only 6 workers exceeded the effective dose of
250mSv and several dozen others received doses higher than
100mSv, which may increase the risk of cancer by only about 1% (from spontaneous 20% to 21%). It
is estimated that several thousand people near Fukushima could
receive doses in the range of 10-30 mSv (as
if they had undergone one X-ray CT scan - irrelevant!). The relevant Japanese
institutions dealt very responsibly with the radiation protection
of the population. The effects of the Fukushima nuclear accident
are only local and do not exceed the severity of
other major non-nuclear industrial accidents. The main health
risks and injuries were caused not by radiation, but by stress
from evacuation and concerns arising from overestimation of risks
- radiophobia; of these causes several hundred
people died, the evacuation was overvalued ...
*) The disinformation
about Fukushimské crash paradoxically contributed very much in
Japan, where this event has been misused to
fight among influential individuals, political and economic
groups, enforcement of their particular interests.
The subjective, biased and even false reports of
the commissions were pushed to the official publication,
claiming, among other things, that the accident was not caused by
a tsunami after the earthquake, but by humans: "It was a
serious man-made disaster be prevented ", in stark contrast
to the facts. And to promote the cessation of nuclear
energy in Japan ... It is debatable whether the severity
and impact of accidents could be mitigated by more efficient
responses and organizational expertise circles ..?...
It for adverse events is a sensible
approach constructively and take them positive lessons
to of the future. The Fukushima accident revealed, among other
things, one important lesson: for inherent
safety, cooling of the residual heat of the reactors
should be provided from water tanks located above the
reactor so that the cooling water flows by gravity -
"self heawy" - and is not tied to pumps that may fail,
eg when flooded.
Some general aspects of radiation
accidents are discussed in §5.6, section "Radiation
accidents and incidents".
Safety and
risks of nuclear energy
Nuclear safety is ensured by a set of technical means and
measures to prevent the process of obtaining nuclear energy from
escaping control and to prevent the emergence of radioactive
substances from entering the environment. The safety of nuclear
facilities is based primarily on the well-thought-out
design of all links in the nuclear chain, with multiple
barriers against unwanted leakage of radioactive substances and
multiple secured regulatory and safety systems. An important
element is also the passive safety of reactors,
given the self-regulatory properties, which in
the case of an anomalous situation will stop or dampen the
fission reaction without human intervention. Inherently
safe nuclear reactors are designed in such a way that in
the event of any failure, the reactor is shut down by the action
of physical laws only, regardless of the operator's activity (lat. in = in, haereo = imprison
; inhaerent = inseparable,
intrinsically connected, innate, essential ). Part of the safety of a nuclear reactor is also to
ensure the cooling of the reactor core, not only
during operation, but also the cooling of residual heat
after shutdown *). The safest reactor is one that does not have
enough fuel to generate an excess of neutrons, so that it cannot
occur in an uncontrolled chain fission reaction under any
circumstances; however, such a reactor must have an independent external
neutron source to operate (see below)ADTT). And of course the thermonuclear reactor
(below the section "Fusion of atomic nuclei"), where no chain reaction takes place.
*) The recent accident at the Fukushima spring power
plant teaches that cooling of the residual heat of the reactors
should be provided from water tanks located above the
reactor so that the cooling water flows by gravity and
is not tied to pumps (which may fail, eg when flooded).
Opinions differ widely on the safety or
"danger" of nuclear energy. While experts generally
agree that nuclear energy is relatively very safe,
there is no such agreement in the lay public. Serious voices of
experts are shouted in the mass media by very agile groups of opponents
of nuclear energy. The sad event that significantly
"encouraged" these activists was the human factor
caused by the tragic accident at the Chernobyl nuclear power
plant (described above in the section
"Chernobyl nuclear reactor
accident"). For political or lobbying reasons, some mass media
inflated this accident to catastrophic proportions and misused it
to fight nuclear energy as such. Recently, the
accident caused by the natural disaster at the Fikushima nuclear
power plant (passage "Accident at the Fukushima nuclear power plant"), which was only an
insignificant episode in the tragic context of a natural disaster
...
The objective problem of nuclear
energy is nuclear waste
(see below) - spent fuel cells containing significant amounts of
radionuclides, some of which have a very long half-life. So far,
these wastes are stored in specially adapted repositories, but
technologies are being developed for their efficient disposal or
further processing (see ADTT below).
Ordinary environmental activists
undoubtedly have good intentions and are
convinced that they are fighting for a better environment.
However, it is mostly lay people who, without real
knowledge of things, "cry on the wrong grave":
they do not realize that the "threads" that actually
control them secretly come from the opposite camp - from economic
lobbies, which for their particular interests and profits are
able to devastate the environment uncontrollably. It is
not generally known, for example, that thermal power plants, in
addition to enormous contamination with sulfur or nitrogen
compounds and other harmful substances, contaminate
the environment with radioactivity, much more
than nuclear power plants! Nuclear energy,
especially the implementation of promising new technologies - in
future nuclear fusion - is the only
reasonable alternative for the present inefficient and
environmentally harmful use of fossil fuels.
However, what can be fully agreed with
"green" ecologists is that the best energy is "saved
energy" - the development of modern technological
processes that have less energy intensity... Some social aspects
of obtaining and using energy are discussed at the end of this
chapter in the passage "Energy-life-society".
Nuclear waste
The usual "charge" of fuel for a (light water) nuclear
reactor with a capacity of 1000 MW is about 100 tons of uranium
enriched to about 3%. One ton of such fuel contains 967 kg of
uranium-238 and 33 kg of uranium-235. After three years of
reactor operation, about 25 kg of uranium-235 and 24 kg of
uranium-238 will "burn" (transmute) one ton of this
fuel. This produces 35 kg of fission products (a number of
different nuclides), about 9 kg of plutonium isotopes, 4.5 kg of
uranium-236 isotope, 0.5 kg of neptunium-237 isotope, 120 g of
americium-243 and a smaller amount of other transurans. From the
point of view of a conventional reactor, all these radionuclides
contained in spent nuclear fuel are nuclear waste.
Thus, one of the main problems of
current nuclear energy is spent nuclear fuel,
which contains high activities of a number of radioisotopes *),
often with a considerably long half - life. Their escape into the
biosphere is a potential risk for a long time. In addition to
relatively short-lived radionuclides (such as 131I with T1/2 8 days), there is a
large amount of eg 137Cs (T1/2 30 years), 90Sr (T1/2 28.8 years), 241Am (T1/2 458 years), 239Pu (T1/2 2.104 years), 240Pu (T1/2 6.103 years) and a number of other long-lived radionuclides.
Spent nuclear fuel from the reactor produces heat for several
years and has shown radioecologically increased
radioactivity for hundreds and thousands of years.
*) In §1.2 "Radioactivity" it
was derived that the activity of a given number
of nuclei is inversely proportional to the half-life of T1/2 . New (fresh, unused) fuel
cells have a relatively low activity due to the
long half-life a decay of uranium (450 million years). However, the
fission of uranium produces radionuclides with significantly shorter
half-lives (of the order of days or years), so that
freshly burned fuel cells are highly radioactive
!
With these hazardous radioactive
waste can be managed in essentially three ways (after a short cooling period and temporary storage in
intermediate storage facilities, as mentioned above) :
Thus, in addition to chemical treatment and
separation of nuclear waste, it offers a very promising
possibility to transmutate the respective
radioisotopes into stable nuclei by means of a series of repeated
neutron absorption *), followed by b-decay or fission.
Unfortunately, however, this neutron absorption in a classical
nuclear reactor proceeds with little efficiency because the
effective cross section of slow neutron capture is very low for
most isotopes and, in addition, most neutrons are consumed to
sustain the chain reaction. Classical nuclear reactors
"struggle" with a neutron balance, neutrons can not
"wasting". For efective transmutation, additional
higher energy neutrons must be used, which can be achieved with
the aid of an accelerator, as outlined below.
*) Possibilities of nuclear transmutations
are also considered with the use of accelerated protons or other
charged particles, but neutron transmutation is probably more
feasible and efficient.
Nuclear
reactor with an external neutron source. Accelerator-controlled
transmutation technology - ADTT
As follows from the above analysis of the possibilities and
current technical solutions for obtaining energy by fission of
heavy atomic nuclei, there are three permanent problems of this
technology :
¨ Nuclear waste with high activity and long half-lives.
¨ Low utilization (burning) of fissile material or primary
fuel.
¨ Reactor safety - a supercritical amount of fuel
(reactivity supply) is required for operation, so there is a
possibility of an accident.
Recently, attempts have been made
that could simultaneously solve all these problems and result in
the construction of a completely new type of nuclear reactor,
which is in terms of fissile material subcritical
and necessary neutron balance for fission is provided by an external
source of neutrons: a fission reactor combined with a powerful
accelerator. This program is called ADTT
(Accelerator Driven Transmutation Technologies *).
*) Sometimes these systems are also referred to as
ADS (Accelerator Driven Systems) -
"accelerator-controlled systems", or more specifically
ATW (Accelerator Transmutation of Waste) -
"accelerator-performed transmutation of waste".
Compared to existing
technologies, the ADTT system would have three main advantages :
In such a reactor with a lower concentration of fissile elements, a separate chain fission reaction is not maintained - the reactor is subcritical, but the supply of missing neutrons is provided by an external source - a powerful proton accelerator that fires nuclei of heavy elements (lead, tungsten, ...) in a target in reactor active zone and the necessary neutrons are ejected from them by the fragmentation reaction - Fig.1.3.5.
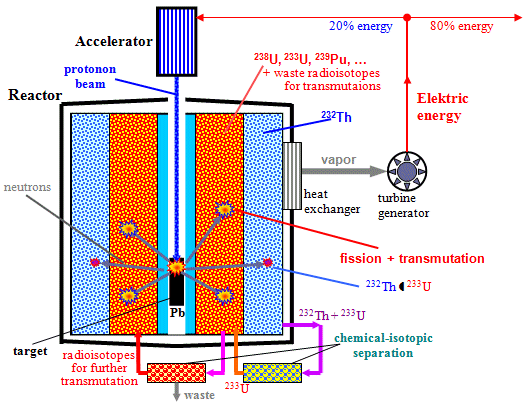 |
Fig.1.3.5. Simplified schematic diagram of a reactor for accelerator-controlled transmutation technology. |
Each proton with an energy of about 1 GeV will
eject about 20-50 neutrons of different energies from the heavy
nucleus, which pass through the basic moderator (eg a layer of heavy water D2O to increase the probability of
fission) into the reactor's own core. Here, the fissile material
as well as the waste isotopes that we want to transmute, would be
dissolved in a suitable environment (molten fluoride salts are
proposed). In the outer part of the reactor, the action of
neutrons could lead to transmutation of thorium-232 by
a series of reactions: n + 232Th ® 233Th + g, 233Th ® 233Pa + e-+n, 233Pa®233U+e-+n. Thorium reserves in the earth's crust minerals are
about 4 times larger than uranium, which could cover human energy
needs for a long time. The resulting uranium-233 would be led to
the central part, where the absorption of neutrons would fission
it to release the relevant nuclear energy. In addition to the
thorium-uranium fuel cycle, a more common uranium-plutonium fuel
cycle can also take place here.
Since the reactor operates
continuously in the subcritical mode, it is operationally safe,
there can be no uncontrolled chain fission reaction - the
reaction rate is determined by the flow of protons from the
accelerator and when it is turned off, the reaction stops.
Series: fission ® transmutation ® b-decays, taking place in the ADTT-reactor would function
both as a source of nuclear energy and as an
efficient neutron "incinerator" for radioactive
waste, where long-lived radionuclides would be gradually
transmuted to short-lived or stable. For neutron transmutation,
radionuclides with a high effective cross section sn of neutron capture are suitable; of long-term
radionuclides in fission products it is mainly :
99 Tc (T 1/2 = 2.1.10 5 years) + n ( s n = 20b) ® 100 Tc (T 1/2 = 16sec.) ® 100 Ru (stable),
129 I (T 1/2 = 15.7.10 6 years) + n ( s n = 18b) ® 130 I (T 1/2 = 12h) ® 130 Xe (stable).
Less suitable is zirconium 93Zr (T1/2 =
1,5.106
years) + n (sn = 2,7b) ® 94Zr (stable), which has an effective neutron reaction
cross section with only sn=2.7 barn; similarly
in 107Pd, 126Sn, 79Se (which, after all, are formed only in small amounts).
An efficient chemical-isotope
separation unit would have to be included in the circuit
of the transmutation reactor, which would continuously separate
the long-lived isotopes and (possibly together with 233U) return them back
to the reactor core. This continuous "on-line"
separation and reprocessing is only possible when using nuclear
fuel in liquid form (melted in fluoride salts,
as mentioned above in the "Salt Reactors"
section). Short-lived and stable isotopes could then be stored in
ordinary storage; their activity would fall to the level of the
natural radioactive background in a few decades.
| Transmutation technology : |
| Successful mastery of transmutation technology would function as a rich source of nuclear energy and at the same time as a neutron "incinerator" for hazardous radioactive waste. |
Electricity would then be produced downstream of the heat exchanger in the secondary circuit by conventional steam turbines. With a technically advanced solution, the accelerator (proton energy approx. 1GeV, flux of tens to hundreds of mA) would consume approx. 20% of the energy produced, the rest could be supplied to the network. After overcoming the technical problems (there are many of them and they are very difficult!), it would be possible in the future to satisfactorily close the nuclear fuel cycle in fission reactors as well. However, unless thermonuclear fusion, discussed below, becomes a more promising way in this direction (section "Fusion of atomic nuclei. Thermonuclear reactions"); that would definitely be a more favorable option! After all, in addition to accelerator-controlled technology, thermonuclear-controlled transmutation technology is also being considered, as discussed below in the "Thermonuclear Fusion" chapter, "Hybrid Fusion-Fission Nuclear Power" section.
T
r a n s u r a n s
At this place, in connection with the heavy fissile materials
discussed above, it is a good opportunity to discuss briefly the
specific issue of the heaviest atomic nuclei. As
transuranium elements are designated elements
that follow uranium in Mendeleyev's periodic table and are
"heavier" than uranium. They are not commonly found in
nature because they are radioactive with a
half-life shorter than that of natural (primary) radionuclides
*). However, they are formed artificially during
some processes in nuclear reactors and during the bombardment of
heavy nuclei with accelerated ions.
*) Transuranic nuclei undoubtedly formed
during the explosion of supernovae similar to
other heavy elements (such as uranium) - see eg "Cosmic alchemy", but due to its relatively short half-lives, has
completely disintegrated over the past billion years and has not
been preserved on Earth or in the solar system.
The names of the first two
transurans - neptunium and plutonium - are
derived from the planets Neptune and Pluto,
whose orbits lie beyond planet Uranus. Another TRU were
named after countries and cities - eg. americium
, californium , moscovium. Many of the TRU was
named after the prominent researchers in the field of
physics and chemistry - eg. einsteinium, mendelejevium,
curium, fermium, ruthefordium, bohrium, copernicum, ... Or
according to researchers directly involved in the discovery
of new transurans - seaborgium, flerovium,
or laboratories involved in the preparation of new
transurans - berkelium, lawrencium, dubnium, darmstadtium.
Lighter transurans
Lighter transurans, such as neptunium, plutonium, americium,
curium, are commonly formed from uranium by neutron
fusion as "by-products" in nuclear reactors.
Because they have relatively long half-lives, we can chemically
extract them from spent nuclear fuel.
In the section on fissile materials, it was
mentioned above how neptunium 237Np and the important
transuranic element plutonium 239Pu
are formed in a nuclear reactor during irradiation of uranium-238
with neutrons. Further irradiation of plutonium with neutrons in
the reactor among other things, reactions occur 239Pu94(n,g)240Pu94(n,g)241Pu94 ®(b-,13 years)® 241Am95 , during which the
following transuranic element americium -241 is
formed (is a-radioactive with a half-life of 458 years). Further
irradiation of plutonium and americium targets with neutrons in
the reactor can also produce some other transuranic isotopes,
such as berkelium Bk or californium Cf. In addition to plutonium
and americium, from a transuranic radionuclides is used californium,
especially 252Cf98 , which, in addition to a-radioactivity, exhibits spontaneous
fission with a half-life of 2.65 years, emitting fission
neutrons - such a radionuclide can serve as an intense laboratory
source of neutrons.
Heavy transurans
Heavier transuranic nuclei (Z>100) can no longer be obtained
by neutron fusion in a nuclear reactor. They can only be created
with the help of accelerators: existing heavy
nuclei are bombarded by other accelerated nuclei
so that during the nuclear reaction they
"fold" or "merge" - fuse,
to form a new superheavy transuranic nucleus. In the simplest
cases, we bombard uranium or
lighter transuranic nuclei with a-particles, ie helium nuclei
(with an energy of about 40 MeV). The classic case is the reaction 239Pu + 4a ® 242Cm + 1n, which in the 1940s G.Seaborg formed from
plutonium-239 the curium 242. In the same way, from
americium-241 were formed berkelium Bk97 and from curium made
the californium 252Cf98 .
For the
preparation of the heaviest transuraniums, irradiation with
particles a (helium nuclei) is no longer sufficient, but it is
necessary to bombard with heavier accelerated nuclei.
We bombarded heavy target nuclei of lead, uranium or lighter
transuranium, with multiply charged ions (eg
carbon C6+,
oxygen, neon, boron) accelerated in cyclotrons to energies
exceeding the value of the Coulomb potential wall for the given
interaction (energies around 120-400 MeV
and higher are used). The first such
successful reaction was carried out in 1958 in Berkeley, when
carbon nuclei were fired at the curium target 244Cm + 12C ® 254No102 + 2n, managed to prove the formation of nobelium
nuclei with a proton number Z = 102. Soon after, the same
laboratory succeeded in obtaining a lawrencium with
Z=103 by bombarding a target from californium with accelerated
boron nuclei.
The formation of the heaviest
transuranics (Z>100, N>250) is technologically and
experimentally highly demanding, mainly for two
reasons :
Compound nuclei, formed during fusion reactions of accelerated nuclei with heavy target nuclei, are usually formed in an energetically excited state. If this excitation is high, the resulting nucleus tends to split spontaneously into two lighter fragments (+neutrons) very quickly - we will not detect the transuranic nucleus. In contrast, at low excitation, the complex nucleus of excess energy is emitted by the emission of only a small number of particles such as neutrons, protons or a-particle; the result may be the desired transuranic core. The successful synthesis of heavy TRU's therefore greatly depends on the proper "tuning" the energy bombarding nuclei only slightly higher than what is needed to overcome the repulsive electric Coulomb barrier, so that it occur the so-called "soft fusion", leading to low excitation compound nucleus.
Fig.1.3.6. Preparation, separation and
analysis of heavy transuraniums.
Above: One
of the older simple arrangements for the production, separation
and detection of intermediate-lived transurans. Expression
analysis is mediated by a fast belt conveyor, on the surface of
which a target layer is applied (modification of this arrangement
was used at the JINR in Dubna in the preparation of transuranium
with Z = 102 and 104) . Bottom: An
electromagnetic velocity filter separator is now
preferably used to identify a small number of short-lived heavy
transuraniums against a large background of other nuclei and
processes (analogous filters are used, for example, in mass
spectrometers). The high-energy ion beam from the accelerator
hits a heavy metal target (lead, bismuth or transuran), where a
number of different nuclear reactions take place. The products of
these reactions are ejected from the target and move at high
speed through the evacuated space. They pass through a system of
deflection electrodes and electromagnets generating such
perpendicular combinations of electric and magnetic fields, that
the electric and magnetic forces cancel each other out at a
certain velocity of the passing ions. These ions pass through a
velocity filter ("useful particles"), while particles
of other velocities are diverted by the magnetic field and leave
(into the absorber). Thus the device using a suitably configured
system electromagnetic field separates the
required radionuclide from other reaction products as well as
from primary particles. Only the nuclei of selected velocities,
corresponding to the kinematics of the desired production
process, fall into the detection system, where their energy,
position, energy of decay products and radiation g from the excited
levels are measured. The studied nuclei fly into a semiconductor
detector, where they are braked ("implanted" into its
material) and the radiation emitted during their subsequent
radioactive transformation (alpha or spontaneous fission) is
registered there. This selection method can significantly reduce
the number of alternative undesirable detected processes, ie
significantly reduce the background and thus
increase the chance of observing rare cases of
production of the desired transuranic nuclei.
Using the methods outlined above, a number of
heavy transurans were created: mendelevium 255-257Md101, nobelium 251-257No102, lawrencium 256,259Lw103, ruthefordium 260Rf104, dubnium Db105, seaborgium Sg106, bohrium Bh107, hassium Hs108, meitnerium Mt109, darmstadtium Dt110, and higher.
Elements with
a proton number greater than 110 have not been named until
recently and were given provisional names and marks derived from
the Latin name of the number of their protons: unununium Uuu111, ununbium Uub112, ununtrium Uut113, ununquartium Uuq114 (also called
ununquadium), ununpentium Uup115, ununhexium Uuh116, ununseptium Uus117, ... In the last published transuran with the
designation ununoctium Uuo118, krypton ions accelerated to 450 MeV in a reaction of 86Kr36 + 208Pb82 ® 293Uuo118 + 1n were fired at the
Berkeley laboratory by bombarding lead nuclei, only 3 cores
detected; half-life < 1ms, the experiment was not entirely conclusive. A
hypothetical element with a proton number of, for example, 126
would have the provisional name unbihexium and the brand Ubh126.
Definitive names and designations of
new elements are assigned by the commission IUPAC
International Union of Pure and Applied
Chemistry, an organization dealing with, among other things,
chemical nomenclature and terminology),
based on the proposals of their discoverers, only after the
definitive demonstration of a new element. Of the super-heavy
transurans, the elements Roentgenium Rg111, Copernicium Cn112, Flerovium FI114 and Livermorium Lv116 have recently been
officially named in this way. The synthesis of elements with
proton numbers 113, 115, 117 and 118 has recently been recognized
as proven by this commission, so their names are now also
established - see table.
Note:
The question may arise, why is the discovery
and recognition of new superheavy elements so
"skipping"? In general, the rule is that the
heavier the nucleus, the more difficult it is to synthesize and
discover. However, there are some exceptions
to this rule, stemming from experimental techniques and from the
nuclear-physical properties of the structure of heavy nuclei and
their nuclear reactions.
Three laboratories are mainly involved in the research of the heaviest transuraniums: Lawrence's Laboratory in Berkeley, JINR in Dubna and GSI in Darmstadt (leading experts and pioneers in the preparation of heavy transuranium were G.T.Seaborg and G.N.Flerov in particular). Although these elements have no practical significance, the search for the heaviest elements at the very limit of stability can be of considerable theoretical importance for understanding the laws of atomic nucleus structure, the properties of nuclear forces, and for verifying and refining the atomic nucleus shell model.
| Brief overview of transuranics : |
| The name of transuran | Half-life T1/2 of the most important isotopes | Production | Discovery |
| Neptunium Np 93 | 237 Np: 2.14.10 6 years, ... | 238 U + 2n ® 237 U ( b - ) ® 237 Np | 1940 |
| Plutonium Pu 94 | 239 Pu: 2.44.10 4 years, ... | 238 U + n ® 239 Pu | Berkeley, 1941 |
| Americium Am 95 | 241 Am: 458 years, ... | 239 Pu + n + n ® 241 Am | Berkeley, 1944 |
| Curium Cm 96 | 246 Cm: 15.6.10 6 y., 248 Cm: 348000 y., ... | 239 Pu + a® 242 Cm | Berkeley, 1944 |
| Berkelium Bk 97 | 247 Bk: 1380 y., 247 Bk: 300 y., ... | 241 Am + a® 243 Bk | Berkeley, 1949 |
| Californium Cf 98 | 251 Cf: 898 y., 249 Cf: 351 y., ... | 242 Cm + a® 245 Cf | Berkeley, 1950 |
| Einsteinium Es 99 | 252 Es: 472 d., 254 Es: 276 d., ... | 238 U + n + ... + n ( b - ) | Berkeley, 1952 |
| Fermium Fm 100 | 257 Fm: 100.5 d., 253 Fm: 3 d., ... | 238 U + 16 O ® 245 Fm | Berkeley, 1952 |
| Mendelejevium Md 101 | 258 Md: 51.5 d., 260 Md: 32 d., ... | 253 Es + a® 258 Md | Berkeley, 1955 |
| Nobelium No 102 | 259 No: 58 min., 253 No: 1.6 min., ... | 244 Cm + 12 C ® 244 No | Berkeley, 1958 |
| Lawrencium Lr 103 | 266 Lr: 11 hrs., 262 Lr: 3.6 hrs., ... | 245 Cf + 10 B ® 257 No | Berkeley, 1961 |
| Rudhefordium Rf 104 | 263 Rf: 10 min., 265 Rf: 1.5 min., ... | 242
Pu + 22 Ne ® 260 Rf 249 Cf + 12 C ® 258 Rf |
SUJV Dubna, 1964 Berkeley, 1969 |
| Dubnium Db 105 | 268 Db: 29 hrs., 270 Db: 23 hrs., ... | 243 Am + 22 Ne ® 260 Db 249 Cf + 14 N ® 260 Db |
SUJV Dubna, 1967 Berkeley, 1970 |
| Seaborgium Sg 106 | 271 Sg: 2 min., 267 Sg: 1.4 min., ... | 249 Cf + 18 O ® 263 Sg | Berkeley + Dubna, 1974 |
| Bohrium Bh 107 | 267 Bh: 17 s., 272 Bh: 10 s., ... | 209 Bi + 54 Cr ® 262 Bh 249 Bk + 22 Ne ® 266 Bh |
SUJV Dubna, 1976 |
| Hassium Hs 108 | 269 Hs: 27 s., 270 Hs: 3.6 s., ... | 208 Pb + 58 Fe ® 265 Hs | GSI, 1984 |
| Meitnerium Mt 109 | 278 Mt: 8 s., 276 Hs: 0.2 s., ... | 209 Bi + 58 Fe ® 266 Mt | GSI, 1982 |
| Darmstadtium Ds 110 | 281 Ds: 10 s., 279 Ds: 0.2 s., ... | 208 Pb + 62 Ni ® 269 Dt | GSI, 1994 |
| Roentgenium Rg 111 | 282 Rg: 2 min., 281 Rg: 17 s., ... | 209 Bi + 64 Ni ® 272 Rg | GSI, 1994 |
| Copernicium Cn 112 | 285 Cn: 29 sec., 283 Cn: 4 sec., ... | 208 Pb + 70 Zn ® 277 Cn | GSI, 1996 |
| Nihonium Nh 113 (formerly Ununtrium Uut) |
286 Nh: 20 sec., 285 Nh: 5 sec. | 287.8 Uup ( a ) ® 283.4 Nh | SUJV April, 2003 |
| Flerovium FI 114 (formerly Ununquadium Uuq) |
289 FI: 2.6sec., 288 FI: 0.8 sec., ... | 244 Pu + 48 Ca ® 291 FI | SUJV Dubna, 1998 |
| Moscovium Mc 115 (formerly Ununpentium Uup) |
285 Mc: 0.22 sec., 288 Mc: 0.088 sec., ... | 241 Am + 48 Ca ® 187.8 Mc | Dubna + Berkeley, 2003 |
| Livermorium Lv 116 (formerly Ununsextium Uus) |
293 Lv: 0.06 sec., 290 Lw: 0.02 sec., ... | 248 Cm + 48 Ca ® 292 Lv | Berkeley + Dubna, 1999 |
| Tennesine Ts 117 (formerly Ununseptium Uus) |
294 Ts: 0.05 sec., 293 Ts: 0.02 sec., ... | Dubna 2010 | |
| Oganesson Og 118 (formerly Ununoctium Uuo) |
294 Og: 0.7 msec. | 208 Pb + 86 Kr ® 293 Og 249 Cf + 48 Ca ® 294 Og |
Dubna, Berkeley 1999,2006 |
Note :
For reasons of space in the column
"Production", the production reaction are write
only very simplified and do not include the particles
emitted during the reaction (usually neutrons, electrons,
or a particles).
|
All TRU disintegrate by a -radioactivity,
the heavier ones also by spontaneous fission.
Alpha-decays, event. combined with b- decays, followed by
several in a row, until this decay chain hits one of the 4
nuclides: thorium 232Th, uranium 238U,
uranium 235U or neptunium 237Np.
Further decay then continues with one of the 4 standard
decay series shown in Fig.1.4.1 in §1.4 "Radionuclides".
E.g. 241Am ® a + 237Np ® 7 a + 4 b + 209Bi - neptunium
decay series; 239Pu ® a + 235U ® 7 a + 4 b + 207Pb - 235U - actinium
decay series; 252Cf ® a + 248Cm ® a + 244Pu ® a + 240U ® b + 240Np ® b + 240Pu ® a + 236U ® a + 232Th ® 6 a + 4 b + 208Pb- thorium
decay series; analogously other transurans.
Chemical properties of
transurans
From the point of view of classical chemistry, it can be expected
that the chemical properties of transurans should correspond to
their position in the Mendeleev periodic table,
determined by proton number Z. For
lighter transuraniums with Z from 93 to 103, it was
verified that their basic chemical properties actually correspond
to their position in the relevant columns of the
periodic table. However, ruthefordium (104) and dubnium (105)
showed differences in chemical behavior (Rf
in solution reacts similarly to plutonium or samarium and Db
exhibits protactinium-like behavior; whereas according to the
periodic table they should behave like the elements hafnium and
tantalum, located just above them).
Seaborgium (106) and bohrium (107) behave as tungsten and rhenium
according to chemical experiments, in concordance with the
periodic table.
We can prepare superheavy transurans
only in very small trace amounts, sometimes only
a few nuclei. They are often very unstable and
break down into lighter elements in a fraction of a second. It is
therefore very difficult to investigate their chemical
properties. The usual method of analytical chemistry cannot be
used here: to put a substance in a test tube and observe how it
reacts with other chemicals, usually in a "wet" way in
solution. The methods of current expression chemical analysis
make it possible to investigate the properties of isotopes with a
lifetime longer than about 1 second. For the newly discovered
most heaies elements, however, it may be an express analytical
"chemistry of one atom".!..
In short-term heavy transurans we
are able to observe fragments of their nuclear decay, which carry
information about the physical properties of
these nuclei. Investigating the chemical properties
of atoms of these elements nuclear physicists and chemists try to
do with complicated experiments. The surface of the target,
maintained at very low temperature, is covered with a coating of
selected chemicals. Depending on the coating to which the
affinity of the resulting atoms of the investigated transuran is
observed, their chemical behavior is judged. Spectrometrically
can measure the properties of the performed chemical reaction (here so far achieved not convincing results...).
Different chemical
properties of heavy transurans ?
It is expected that the principle of similar behavior of elements
in the same column of the periodic table may be disrupted
for very heavy atoms due to relativistic effects.
With a high number of protons, the electric charge of the nucleus
is high, which also leads to a high velocity of electrons on the
internal orbitals. In heavy transuranics, the inner electrons
reach orbital velocities that are already approaching the speed
of light (they become "relativistic"), so the effects
of the special theory of relativity are beginning to apply here.
The inertial mass of the electrons increases, which (together
with the relativistic contraction of the lengths) causes a decrease
in the size (shrinkage) of the internal orbitals
*).
*) At Z> 170, electron collapse
could even occur from the K-shell to the nucleus (there it
combines with protons - cf. §1.2, passage "Electron capture (EC)"), so these superheavy nuclei (if they can exist
at all?) probably can't form atoms...
Reducing the radius of the inner
orbitals results in an increase in the electrical
"shielding" of the positive charge of the nucleus by
these electrons, so that more distant electrons (no longer
relativistic) are attracted to the nucleus by less force.
External orbitals, especially valence ones, are less bound to
heavy atoms than would correspond to a conventional
non-relativistic quantum model of an atom. And also the energy
distance between the external levels is smaller. There is less
electrical cohesion between the nucleus and the shell of less
ionizing energy for the electrons in the outer valence shell. The
relativistic quantum mechanical effects thus cause changes in the
structure of the atomic shell, whereby the atoms of very heavy
elements may behave chemically differently than
we would assume based on their proton number and location in
Mendeleev's periodic table.
E.g. the last known transuran oganesson
(Z = 118) is included
in the noble gas column; therefore, it also received the
traditional suffix "on", it was assumed that
it would be another even heavier radioactive gas after radon.
However, due to the above differences, it is expected that it
will actually have a higher reactivity, it will not be an inert
gas, but will also have metallic properties and form oxides and
halides ...
If other even heavier
transurans are discovered, their proton numbers will
only be formal, as their chemical properties will
be different, not corresponding to the position in the
relevant column of the table..! .. From the point of view of
chemistry, however, this is irrelevant, because these superheavy
nuclei decay so fast, that their volatile atoms are not enough to
form any compounds...
In §1.1, section "Structure
of the atomic nucleus" and §1.2,
passage "Stability and instability of atomic
nuclei", it was analyzed that the
stability or instability of atomic nuclei is determined by a
complex interplay of strong, weak and electromagnetic
interactions inside the nuclei, related to the number of protons
and neutrons, their mutual ratio and arrangement in the shell
model of the nucleus. Protons and neutrons inside the nucleus
occupy energy "shells", each of which contains a
certain number of particles. A shell is more stable when it is
completely filled - it contains the so-called "magic"
number of particles. Overall, however, it is observed that
very heavy nuclei have a greater tendency to decay, in connection
with the short range of the strong nuclear interaction.
"Island of
stability" of heavy transuranic nuclei ?
The heavier the transuranic nucleus, the shorter its half-life is
usually observed *). However, some experts assume that for even
heavier nuclei the the half-life will start to temporarily lengthen
again - that there could be a kind of "island of
stability" (relative
stability, however) in the region of
superheavy nuclei. However, this is still only a hypothesis based
only on the extrapolation of the magic numbers
2, 8, 20, 28, 50, 82 and 126, corresponding to filled proton or
neutron shells in the nucleus, which results in a configuration
with increased stability. Increased stability is
expected especially for "doubly magic" nuclei for both
protons and neutrons. A nucleus with 126 protons and 184 neutrons
could perhaps form the center of this hypothetical island of
increased stability..?..
*) The half-life also depends significantly
on the number of neutrons in the nucleus, i.e. on the
transuranium isotope. If an isotope with
a longer half-life can be prepared, it may facilitate
the analysis of the physical and chemical properties of this
transuranium element.
There is also speculation that some
heavy transuraniums with twice the magic number of protons and
neutrons could even be practically stable or long-lived.
However, due to the short range of the strong nuclear
interaction, these superheavy nuclei will in any case be considerably
unstable due to alpha radioactivity and spontaneous
fission; therefore, no significant "island
of stability" can be expected..?..
Fusion
of atomic nuclei. Thermonuclear reactions.
The second way (as opposed to the fission
discussed above) to obtain energy in
nuclear reactions, is the synthesis -
connection, merging, fusion - of light element nuclei
into heavier elements. A large amount of binding energy is
released, because medium-heavy nuclei have a much higher binding
energy of nucleons than light nuclei, see a very steep increase
in the binding energy curve in Fig.1.3.3 (this
picture is presented here again for clarity)
in the area of light nuclei :

Fig.1.3.3. Dependence of the mean binding energy Eb/one nucleon on the
nucleon number N of the nucleus. In the initial part of
the graph, the scale on the horizontal axis is slightly stretched
to better see the differences in binding energy for the lightest
nuclei. The right part schematically shows both ways of releasing
the binding energy: the splitting of the heavy nucleus and the
merging of the two light nuclei.
The most energy efficient and at the same time the easiest to implement (with the lowest activation energy) are fusions of light nuclei 1H, 2H, 3H, 3He, 6Li, in which a helium nucleus 4He is usually formed, which has a particularly high binding energy between light nuclei, see the ascending part of the graph in Fig.1.3.3. There are several reactions to the synthesis of the lightest nuclei.
The most basic is the hydrogen proton-proton
fusion reaction of 1H1
:
1st partial reaction: 1H1
+ 1H1 ® 2He2 + g ; 2He2 ® 2D1 + e+ + n (+ 1,44 MeV) ; e+ + e-® 2g (+ 1,02 MeV)
2nd partial reaction: 2D1
+ 1H1 ® 3He2 + g (+ 5,49 MeV)
3rd partial reaction: 3He2 + 3He2 ® 4He2 + 2 1H1 (+ 12,85 MeV)
As a result, helium is produced. Total energy
balance: 26.2 MeV release = 4.2x10-12 J/(1 He nucleus). p-p reaction is the basis of
thermonuclear reactions in main sequence stars ("Thermonuclear reaction in the interior of stars"). For the first stage of
this reaction, to produce a neutron, the conversion of the
quark "u" -> "d" via the weak
interaction - and therefore with a very low effective cross
section - is required. With huge volumes of hydrogen
"fuel" in the central part of the star, however, even
this low efficiency is sufficient to generate a very high total
energy for the star's luminosity.
In our laboratory conditions, we
do not have such huge volumes and concentrations of
nuclear "fuel", so the p-p reaction is not
applicable here. Similarly, the CNO cycle applicable to
stars of the 2nd and higher generations is not applicable. The
only option is to use "combination" reactions of
already finished higher isotopes of hydrogen (deuterium, tritium,
possibly also with lithium), taking place through strong
interaction.
Further fusion reactions of hydrogen
isotopes, taking place through the strong interaction, are thus :
| 2H1 + 2H1 ® 3He2 (0.8MeV) + 1n0 (2.5MeV) | Þ total yield | 3.13 MeV |
| 2H1 + 2H1 ® 3H1 (1.0MeV) + 1H1 (3.0MeV) | Þ total yield | 4.03 MeV |
| 2H1 + 3H1 ® 4He2 (3,5MeV) + 1n0 (14.1MeV) | Þ total yield | 17.6 MeV |
| 1H1 + 3H1 ® 4He2 (19.9MeV) | Þ total yield | 19.9 MeV |
| 2H1 + 6Li3 ® 4He2 (11.2MeV) + 4He2 (11.2MeV) | Þ total yield | 22.4 MeV |
For energy use, the most promising reaction so
far is between deuterium (D º
2H1) and tritium (T º 3H1)
:
2H1 + 3H1
® 4He2 + 1n0 + 17.6 MeV ,
which takes place most easily of all (at the lowest energy ~
temperature) and releases a considerable amount of energy; the
released energy is carried away in the form of their kinetic
energy by neutrons (14.1 MeV) and helium nuclei (3.5 MeV).
Compared to nuclear fission, the
nuclear fusion has great principal advantages :
If we can effectively manage the process of
thermonuclear fusion and be able to use it energetically,
humanity will forever get rid of its dependence on fossil fuels
and gain access to a practically unlimited source of clean
(low-emission, carbon-free) energy.
The only downside
is that we
are not able to do that yet make..!..
- at least not in a way that can be used for energy production.
Thermonuclear
reactions in stars <-- versus --> in terrestrial
conditions: 10 times higher temperature than in the stars !
We try to imitate the production of energy in the stars
to some extent (it is analyzed in detail in
§4.1 "Gravity and evolution of stars", part "Evolution of stars" - "Thermonuclear reactions inside
stars" monograph "Gravity, black holes and space-time
physics").
In the interior of stars, during their formation (from a protostar), the first
thermonuclear reactions can take place at temperatures of about 1
million degrees. The main thermonuclear reactions of the
synthesis of hydrogen nuclei to helium then have been going on
for many millions and billions of years at temperatures around 10
million degrees. This is due to the fact that the massive gravity
compresses huge masses of thermonuclear "fuel",
hydrogen, to high densities and during this adiabatic compression
it heats them to temperatures around 106 degrees, at which these densities fusions of hydrogen
nuclei with low volume intensity (cca 300
W/m3), but sufficient overall intensity can already take
place to cover the vast radiant energy of stars.
However, in our terrestrial
conditions, we do not have the massive gravity or accumulation of
huge amounts of thermonuclear fuel, so we must
"overcome" the conditions in the stars - reach ten
times the temperature than inside the stars (see also the discussion "Thermonuclear
fusion inside the stars"
below), so that in our small volumes of
sparse plasma, sufficiently intense thermonuclear fusions
took place for energy use. That's why it's so difficult!
Below we will show the ways in which we try to realize this
difficult task in terrestrial laboratories ...
Thermonuclear
reactions
How to realize core merging? In order for the two nuclei to
merge, they must approach each other at a
distance of »10-13 cm, where attractive nuclear forces begin to act. In
doing so, they must overcome the Coulomb electric repulsive
forces acting between consistently positively charged nuclei,
which they can realize only by their accelerating to large
kinetic energies - by supplying high activation energy.
For experimental purposes, this can be achieved with an
accelerator (eg bombarding a tritium target
with accelerated deuterons), but the amount
of nuclei thus combined will be negligible and most of the
supplied kinetic energy of the accelerated beam will be converted
into a heat which heats the target (due to
electrical Coulomb collisions, which are much more likely than
nuclear collisions); the input energy will
always be significantly higher (by many
orders of magnitude!) than the output
energy (obtained). To carry out nuclear fusion and obtain nuclear
energy on a macroscopic scale, there is only one way to achieve
the required activation energy: to carry out the
reaction at a very high temperature - hence the
name thermonuclear reaction. Heating the fuel to
a sufficiently high temperature will cause the kinetic
energy of the thermal motion of the atoms of the
reacting fuel to increase to such an extent that it is sufficient
to overcome the electrostatic repulsive barrier
between the fuel nuclei and the synthesis of the nuclei can take
place due to the attractive strong nuclear interaction (so far, hypothetical alternatives are briefly discussed
below in the section "Alternatives to Nuclear Fusion").
Temperature for realization of
thermonuclear fusion
In order for a nuclear reaction to occur,
the nuclei must approach each other at a distance rs » 10-13 cm, where attractive strong nuclear interactions
begin to act. This requires a relatively high kinetic energy EC which overcomes electrostatic
repulsion barrier (Coulomb potential "hump")
between two nuclei with charge Z1.e and Z2.e : EC = Z1.Z2.e2/rs . Between two hydrogen nuclei with the proton number Z1=Z2=1 there will be a
barrier height EC » 1MeV. A
temperature higher than 1010 degrees would be required to thermally reach such a
value of the mean kinetic energy of the nuclei. Achieving such a
high temperature in the macroscopic volume of plasma in
laboratory terrestrial conditions is not realistic. However,
there are two favorable circumstances that significantly reduce
the minimum temperature required for the effective
formation of fusion reactions :
1. Tunnel effect
, thanks to which there is always a certain non-zero probability
that the Coulomb barrier will be overcome by a particle whose
energy is lower than EC (this
probability of overcoming for a particle with energy E is
approximately PE ~ exp[-Ö(EC/E)] ); see §1.1, section "Quantum
nature of the microworld", passage
"Quantum tunneling").
2. Maxwell statistical
distribution *) at the thermal motion of the particles
shows that there is always a certain number of particles moving
at substantially higher speeds than the mean kinetic energy <ET> = 3/2 .kT.
*) Maxwell-Boltzmann statistical
distribution of thermal motion of particles
Particles of idealized
"gas" in this case, hydrogen ions of mass m ,
is constantly moving, and colides, each of which has a different
instantaneous speed v , direction of movement and
different kinetic energy E = m.v2/2, which are randomly
and erratically changing due to collisions. The distribution of velocities and
energies of random motion of ideal gas particles is described by
the so-called Maxwell-Boltzmann
distribution function P , determining the probability of the
number of particles in the state with velocity v :
P(v) = 4p.(m/2pkT)3/2.v2.exp(-mv2/2kT), or equivalent
with energy E
: P(E) = 2p.(1/2pkT)3/2.v2.exp(-E/kT),
where T is
the thermodynamic temperature and k is the Boltzmann
constant
(expressing the relationship between temperature and energy of
gas particles: it is the amount of kinetic energy of one
particle, which corresponds to a change in gas temperature by 1
°K; it has the value k = 1.38.10-23 JK-1). The graph of this distribution
function is a wide "bell-shaped" (but asymmetrical)
curve, the shape of which depends on the temperature: the higher
the temperature, the wider the shape of the curve and its maximum
is shifted towards higher energies and velocities. The maximum of
the curve determines the most probable velocity vp = Ö (2kT/m), but from a physical
point of view the more important is the mean
square velocity of particles vk
= Ö (3kT/m), which corresponds to the mean kinetic energy of particles at temperature T: <E T > = 3/2.kT.
When converted to nuclear power units [eV], the mean energy of
1eV particles corresponds to a temperature of 11600 °K, so that
a temperature of 1keV represents 11.6 million degrees. With temperature T ,
not only does the mean value of velocity or energy <E T > increase,
but also the relative proportion of
particles with high velocity and energy E >> <E T > increases.
Due to these two circumstances (and under the conditions of a high effective
cross-section of the respective reactions), even in sparse plasma, thermonuclear
reactions between the lightest nuclei can take place relatively
efficiently from temperatures of »1.5.108 degrees.
Analogy of chemical
combustion and thermonuclear fusion
Nuclear fusion is a
"nuclear analogy" of chemical fusion of atoms, such as
conventional combustion (fusion with oxygen).
The fire is ignited only when the external supply (activation)
energy reaches the necessary ignition temperature,
the kinetic energy of atoms overcome the barriers of the
electrical repulsive forces and the atoms closer
together so they may be sharing valence electrons and form electro-chemical
linkage (as discussed in §1.1, section "Interaction of atoms"). This releases the bond energy; if it is higher
than the supplied energy, the reaction is exothermic
and can already maintain the required temperature on its own -
burning continues. The frequency of individual reactions is so
high that the energy released is sufficient to cover the energy
losses in the system.
Similarly, to ignite nuclear fusion, it is
necessary to first supply the activation energy - to
reach a high temperature, in this case almost a
million times higher than with chemical combustion. On the other
hand, the energy released during nuclear fusion is more than a
million times higher than during chemical fusion. If at least
part of this released energy is kept in the reaction
space, the required high temperature can be maintained
and the "fusion combustion" can continue. Due to the
high energy efficiency, nuclear fusion suffices with incomparably
less "fuel" than fire and produces only a small amount
of "flue gas".
The composition and pressure of the
atmosphere here on Earth create suitable conditions for chemical
combustion, which can lead to the risk of fire or explosion.
However, for nuclear fusion, there are natural conditions only in
the center of the stars (see below "Thermonuclear
reactions in stars", or "Thermonuclear reactions inside stars"). Under terrestrial
conditions, nuclear fusion "combustion" is very
difficult to perform, in high-temperature plasma - either
isolated by a magnetic field or briefly created by intense
irradiation. This difficulty, on the other hand, ensures that controlled
nuclear fusion is a safe process. If the fusion reaction
comes into contact with the substance under terrestrial
conditions, it cools immediately and stops (a special exception is the uncontrolled explosive
thermonuclear fusion mentioned below, which, however, can only be
induced in terrestrial conditions by an explosive fission nuclear
reaction; an explosion on a macroscopic scale cannot occur with
the energetically use of fusion).
The reacting
deuterium and tritium (thermonuclear
"fuel") must therefore be heated
to a temperature of min. »108 degrees. At such a temperature, each substance is in a
state of fully ionized plasma - all atoms are
decomposed into free electrons and bare nuclei; these nuclei can
then collided sharply and merge with each other.
Plasma - 4th state of matter
At high temperatures, in an electric discharge or by the action
of ionizing radiation, electrons are ejected from the gas atoms
and the atoms themselves become positive ions. Such a partially
or fully ionized gas is called plasma (Greek plasma = formable, malleable material;
the electric discharge copies the shape of the tube and its shape
is easily influenced by electric and magnetic fields). Plasma is sometimes referred to as the 4th
state of matter (1st solid, 2nd liquid, 3rd gas, 4th
plasma). In order to distinguish this ionized substance from
other situations with electrically charged particles, we require
two additional properties in the physical definition of plasma :
- Electrical neutrality on a
macroscopic scale (on average the same number of electrons and
positive ions) - we do not consider charged particle beams to be
plasma;
- Collective behavior caused by a
long-range interaction of sufficiently close charged particles -
it is not a very dilute or weakly ionized gas by plasma.
Thus, the general physical definition of
plasma is: "Plasma is a set of particles with free
charge carriers that is globally neutral and exhibits collective
behavior". This definition also includes exotic
forms of the substance, such as quark-gluon plasma (§1.5, passage "Quark-gluon plasma -"5th state of
matter" ").
Plasma has significant electrical properties:
it is electrically conductive, it reacts to a magnetic field, it
can generate electric and magnetic fields on its own, complex
electro- and magneto-dynamic processes take place in it. It is
these phenomena that are very important for achieving the
conditions of the thermonuclear fusion described below in tokamaks.
In ordinary terrestrial nature, plasma
occurs relatively rarely in atmospheric discharges, lightning.
From a global perspective, however, plasma is a very important
form of matter - most of the observed substance in the
universe is in the plasma state.
Plasma is characterized mainly by two
quantities :
- Plasma
density n , which is the number
of ions in a unit of volume [m-3].
- Plasma
temperature T, measured either
in the temperature scale [°C], [°K], or equivalent in units of
kinetic energy [eV], [keV] of particle motion. The mean energy of
1eV particles corresponds to a temperature of 11600 °K, so that
the temperature of 1 keV is 11.6 million degrees.
Electrons and ions in neutral plasma act
on each other by electric Coulomb forces. As the electrons move
relative to the ions, the Coulomb force will pull them back,
acting as a "return force". In the field of electric
forces, electrons and ions can perform longitudinal harmonics
- plasma oscillations, the frequency of which
(circular frequency w = 2p f) depends on the plasma density n, on the charge
and on the mass of the particles. Electron plasma
frequency wpe = (n.e2/me.eo)1/2 is significantly higher than the ion plasma
frequency wpi = (n.e2/mi.eo)1/2, because the mass of ions mi is at least 1750 times greater lower than the mass of
electrons me.
The situation where the plasma is in a magnetic
field is important for carrying out a controlled
thermonuclear fusion. When charged particles move, a Lorentz
force acts on them here perpendicular to the direction of
movement, as a result of which the particles they rotate
around magnetic field lines - they perform so-called gyrooscillations
or cyclotron oscillations with the Larmor
frequency given by the charge of the particle q, its mass m
and the intensity of the magnetic field B: wc = q.B/m. The electron
cyclotron frequency wce = e.B/me is again
significantly higher than the ionic cyclotron frequency
wci = e.B/mi .
In addition to these "pure" oscillations
and frequencies, "mixed" so-called hybrid
oscillations are also considered in plasma physics, which
are the result of the interaction between the oscillations of
ions and electrons. The lower hybrid frequency wLH
= [(wci.wce)-1 + wpi-2]-1/2
is a combination of the cyclotron ionic frequency wci and electron wce (their geometric
diameter) and the ion plasma frequency wpi (at stronger
magnetic fields, the contribution wpi is negligibly small). Upper
hybrid frequency wUH = (wpe2 + wce2]1/2 is a quadratic combination of electron
plasma wpe and cyclotron wce frequency.
Language note: The
word "plasma" has two main
meanings: 1. Physical - ionized gas; 2. Biological
- a component of blood (blood plasma), in cells the protoplasm or
cytoplasm.
Explosive thermonuclear reactions
Similar to fission nuclear reactions, fusion thermonuclear
reactions can take place in an uncontrolled
(explosive) or controlled (steady)
manner. Unlike the fission of heavy nuclei
discussed above, thermonuclear fusion does not result in
a chain reaction because the heat and pressure produced
are not sufficient to trigger further fusion (except perhaps the thermonuclear chain reaction in a
nova explosion where strong gravity interacts - see "The role of
gravity in and evolution of stars",
section"Thermonuclear reactions"). The conditions for a
nuclear fusion must be ensured from the outside:
high temperature and pressure + maintenance of high-temperature
plasma in the reaction volume for a sufficiently long time -
either inertially by explosion or by a strong magnetic field (see
below), or gravity in the stars.
Fusion thermonuclear
weapons
Uncontrolled explosive thermonuclear reaction is
the essence of the misuse of nuclear fusion in thermonuclear
weapons, also called the "hydrogen bombs"
(there is a fusion reaction of hydrogen
isotopes - deuterium and tritium). We
cannot carry out a "pure" thermonuclear explosion in
terrestrial conditions (perhaps with the
exception of the "thermonuclear microexplosion" of a
small D-T capsule using powerful laser irradiation - see "Inertial Fusion" below). The energy of a fission
nuclear charge must be used to compress and heat the
thermonuclear fuel to the fusion temperature.
The fission charge and the fusion material
are placed right next to each other inside the solid envelope (pictured right). A mixture of
tritium and deuterium (most often formed from a compound of
lithium and deuterium LiD) with a nuclear
detonator (explosive fission reaction 235U or 239Pu - actually by the
explosion of a smaller "atomic bomb" - was described
above in the section "Atomic nuclear fission", passage
"Uncontrolled chain
reaction - nuclear explosion") compresses
rapidly and heats to a temperature of about 100
million degrees, which causes an explosive thermonuclear
reaction for releasing many times more energy than a
fission "atomic bomb".
| Basic principles of construction with the activities of fissile and thermonuclear nuclear weapons | ||
 |
||
| Left: Fission nuclear bomb (two different constructions) Right: Thermonuclear bomb |
The first experimental thermonuclear explosions
used a mixture of liquid deuterium and tritium, which is not
usable for military purposes. In military thermonuclear warheads,
a compound (hydride - deuteride) of the lighter isotope lithium
with deuterium 6Li 2H is used as an explosive
"charge" and plutonium as a detonator. When the
detonator explodes by chain fission of plutonium, a large amount
of neutrons is formed, which in lithium deuteride react 6Li (n, a )3H converts lithium to
tritium. At the same time, the energy released by the nuclear
fission of the igniter compresses and heats the resulting
reaction mixture to the temperature required for the subsequent
D+T fusion. The first thermonuclear bomb was developed in 1951 by
E.Teller and S.Ulam with collaborators in nuclear laboratories in
Los Alamos.
The design of thermonuclear warheads was
later improved to a three-layer system. In addition to 6Li 2H and plutonium, they
also contain a mixture of gaseous deuterium and tritium
surrounded by plutonium and beryllium in the upper layer. In the
center of the fission stage is a small amount of
deuterium-tritium gas, which during the explosion produces
neutrons by fusion reaction, inducing more efficient fission of
uranium or plutonium ("boosting") in this first degree. The thermonuclear stage then
includes a housing made of uranium-238 ("depleted uranium") and
a fissile 239Pu rod. This is because thermonuclear fusion produces a
huge amount of high-energy neutrons, which are able to fission 238U (which is not otherwise a fission material for slow
neutrons), which further increases the
energy yield of the explosion; however, it also causes large radioactive
contamination with fission products ...
A special variant of
a thermonuclear weapon is the so-called neutron bomb *) , which uses penetrating
neutron radiation, created by the explosion of a small
thermonuclear charge.
*) Neutron weapon: The miniature
explosive thermonuclear reaction was designed for military abuse
in the so-called neutron bomb. LiD (a
solid-state lithium-deuterium compound) is most commonly used as
a thermonuclear explosive, and 239-plutonium is a fissile
detonator. The addition of beryllium leads to the intensive
production of neutrons by reactions (a, n). It was designed as a
tactical radiation weapon against "living force", with
a suppressed destructive effect.
Fortunately,
destructive thermonuclear weapons have never been used in
military operations. However, incomparably more powerful
thermonuclear explosions (compared to which
our nuclear weapons are just "baby capsules") occur in universe. It's in the
so-called nova and supernova explosion - see §4.1 "The role of gravity in the formation
and evolution of stars", section
"Thermonuclear reactions", passage "Late stages of stellar
evolution", in the book "Gravity,
black holes and space-time physics".
Controlled
thermonuclear reaction
Peaceful use of thermonuclear energy is possible only if controlled
thermonuclear fusion will be realized - to construct a thermonuclear
reactor. In order for such a thermonuclear reaction to
take place, two basic conditions need to be ensured :
Conditions for Sustaining Nuclear Fusion and
Positive Energy Yield - Lawson's Criterion
For energy recovery, we need to achieve an energy
gain - that the thermonuclear reaction produce more
energy than it needs to generate and heat plasma and compensate
for energy losses by radiation and plasma leakage. Or, that the
fusion power Pf exceeds the required energy input P: Pf/P ³ 1. For the
successful course and use of thermonuclear fusion, certain very demanding
criteria must be met for plasma density,
temperature and holding time of
sufficient temperature to ignite the fusion and for the duration
of the reaction, the existence of hot plasma was not dependent on
external heating; this is a basic prerequisite for a fusion
reactor with a positive energy yield.
Consider the unit volume (eg 1cm3) of a
high-temperature plasma in an active thermonuclear zone of
temperature T , containing the number n1 and n2 of both light nuclei (mostly deuterium and tritium, ie n1 = nd
, n2 = nt) prepared
in volume unit for fusion. Under equilibrium, the ion density is
equal to the electron density. Total energy density W the
kinetic energies of electrons and ions are W = 3n.kB.T, where kB is the Boltzman
constant and n is the total particle density. For the
density of the particles are in relation n1.n2 = 1/4
.n2 for a
mixture of deuterium and tritium and n1.n2 = 1/2
.n2 for
pure deuterium plasma.
In principle, the following important
phenomena will take place in this test unit volume :
J Fusion
reaction with volume rate (density, reactivity) f = n1.n2.<v. s>, at which nuclear
fusion energy with (density of) power Pf = n1.n2 . <v.s>. ef is released, where v is the
average collision rate of light nuclei, s (T) is the nuclear
effective cross section of the fusion reaction at temperature T
and ef is the energy released in one fusion reaction; <>
denotes the averaging over the Maxwell velocity distribution at
temperature T.
L Energy
losses of Pdam in the plasma cloud due to two mechanisms :
1. Photon radiation
by electrical interactions of electrons - bremsstrahlung
(§1.6, part "Interaction of charged particles") or cyclotron radiation
(in the presence of a magnetic field). In the inner parts of the
plasma cloud, this photon radiation is re-absorbed. The photon
radiation of a hot plasma cloud (its power) is given by the
approximate relation Prad = 1.4.10-34.n e2 .T1/2 [W/cm3], where ne is the number of electrons in a unit volume (electron density of the plasma ).
2. Leakage of particles
from the active zone - transfer of kinetic energy of plasma
particles (especially electrons) to the environment, heat
conduction, with power density Pcond
(when analyzing the inner regions of the plasma cloud, it is
usually neglected, but in the peripheral parts it is crucial). In the most commonly used Deuterium+Tritium fusion
reaction, considerable energy is also carried away by fast
neutrons, which no an electric charge and are not held back by a
magnetic field. However, these neutrons can subsequently be used
to obtain thermal energy and to produce tritium in reaction with
lithium.
Energy losses cause the plasma temperature
to drop rapidly if energy is not replenished and the fusion
reaction to stop. The holding time tE [s] of the plasma energy (at a temperature higher than the
critical fusion temperature) depends on the energy losses Pdam from the plasma
cloud. If these losses are not compensated, tE ~ W/Pdam .
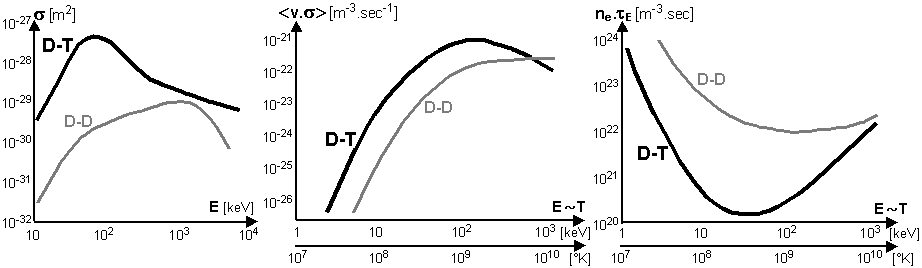
Fig.1.3.7. Dependence of some important quantities for
thermonuclear fusion D-T and D-D on energy E ~ plasma temperature T.
Left: Interaction effective cross
section s of thermonuclear reactions depending on the kinetic energy
of nuclei. Middle: Dependence of the
mean value of the parameter <v. s > fusion reactivity
(reaction rate) on plasma temperature. Right:
Temperature dependence of the minimum value of the product of the
plasma electron density ne and the holding time tE according to the Lawson criterion; value of n.tE the plasma must lie above the plotted curve of the
given reaction in order for the fusion self-heating to exceed the
energy loss.
To maintain the fusion reaction,
part of the energy obtained Pf must be used to heat the plasma and compensate for
energy losses by radiation and conduction to satisfy condition h.(Pf + Prad + Pcond) l Prad + Pcond , where h is the efficiency
conversion of all three forms of energy to plasma heating.
Thermal energy in plasma can be supplemented by two mechanisms :
a) Fusion self-heating directly from the
products of fusion - 100% -absorbed absorbed kinetic energy Eq of released charged
particles, especially alpha - helium nuclei (neutrons escape from the core and do not contribute to
plasma self-heating): f.Eq. For the
deuterium-tritium reaction, Eq = 3.5 MeV, for D-D fusion, Eq
= 1MeV. Self-sustaining
fusion reaction occurs when fusion heat exceed the loss
of energy in the plasma F.eq ³ Pdam, which for D-T
fusion makes: 1/4
.n2 <v.
s >
.E q ³ 3n.kB,T/tE; this can be rewritten in the form of an inequality for
the product plasma density and holding time of its
supercritical temperature: n .tE ³ (12/Eq). (kB T)/<v. s > º L (L is the Lawson
factor). This is one of the three variants of the so-called Lawson's
criterion *) of the sustainability of thermonuclear
fusion. It is graphically shown in Fig.1.3.7 on the right. Size T/<v.s> is a function of
temperature and for the D-T reaction it has a minimum at a
temperature of about 25 keV (300 million degrees), which gives
the numerical Lawson criterion n.tE ³ »1.5.1020 s/m3.
*) A detailed analysis of the kinetics and energy balance
of thermonuclear fusion in 1955-57 was mainly dealt with by J.D.Lawson,
who derived important requirements for the formation and
maintenance of thermonuclear fusion, now called Lawson's
criterion (similar results were reached independently by
Soviet experts P.L.Kapica, L.A.Arcimovic and col.). It requires
that the energy obtained by the fusion be greater than the energy
expended in creating and maintaining the fusion, by means of the
minimum product of the density of the thermonuclear fuel and the
time for which the density and temperature are maintained at the
value required for the fusion to proceed.
b) Electric
heating supplied by recirculation of part of the
electricity produced in the reactor (described
below in the section "Tokamaks"). With the total efficiency h of the conversion of individual forms of
energy into plasma heating, the Lawson criterion has the final
form: n.tE l 3kB.T/a.{[h/(1-h)].<v.s>.ef - T1/2}, where the coefficient a=1/4 for
a mixture of deuterium tritium, a=1/2 for pure deuterium, at the
same concentration of nuclei n.
For a more complex analysis of the fusion reaction, it
is preferred that the Lawson product is explicitly
temperature of plasma T is also included. Thus, the "triple"
product L (Lawson's)
of plasma density n (number of ions/m3), its temperature T (measured either in temperature degrees Kelvin or
Celsius, or in kinetic energy of particles [keV]) and time tE [s ] maintaining its
supercritical energy :
L = n. t E .T ³ (12 / E q ). (k B T 2 ) / <v. s > = c crit ,
which must be higher than a certain critical value ccrit - generalized Lawson's
criterion. For the D-T reaction, the minimum of the triple
product is around 14 keV, which gives the numerical Lawson
criterion n.T.tE l »3.1021
keV.s/m3.
All these conditions are only approximate and idealized.
For the D-T reaction, the value of ccrit
~5.1021 s.keV.m-3, assuming that the
released thermonuclear energy (vs. loss energy of radiation and
escaping particles) is returned to the plasma with an efficiency
of about 33%. At ion temperatures T » 2.108 °C, for D-T reaction, the product of plasma density
and retention time must be n.TE ³ 0.5.1020 m-3 s .
For example, at a plasma temperature
significantly lower than about 108 °K, the frequency of fusion reactions would be too low
at the densities used, at higher temperatures (>200 million
degrees) the energy losses from the plasma increase
significantly.
Lawson's criteria place drastic
limitations on the ability to achieve energetically positive
thermonuclear fusion - in terms of our capabilities, these are extreme
requirements. Above all, it is difficult to reach very
high temperatures of hundreds of millions of degrees,
5-6 orders of magnitude above the ignition temperature of
chemical fuels. No material can withstand these temperatures, so
high-temperature fusion fuel needs to be thermally insulated complex
physical methods described below. Furthermore, it is necessary to
achieve a sufficiently long time maintenance of
the supercritical plasma temperature against energy losses and
plasma scattering. Unfortunately, we do not have the powerful gravitational
forces that control fusion in stars (see
"Thermonuclear reactions in stars" below) . And our technical
capabilities are not yet sufficient for the
energy use of thermonuclear fusion..!.. Lawson's criteria have not
yet been met for existing thermonuclear plants,
even though the JET instrument is approaching them. They should
be met by the forthcoming ITER tokamak (see
below).
Thus, in order to carry out a "successful"
thermonuclear fusion, it is necessary, in addition to a
sufficient temperature T, in principle to achieve a
sufficiently high product of the plasma density
and the holding time n.TE > ccrit . According to
this criterion, it is not necessary to achieve high values for
both parameters n and TE, it is sufficient to
focus on the achievement of a sufficient value for one of them :
- Achieving high values of density of plasma
is oriented technique inertial confinement of
plasma using concentrated energy pulses of the laser, by which
the plasma is pressed to n »1029 cm3 -
for a short time. The holding time is very short tE » 10-9 s, it would work in
pulse mode.
- To prolong the holding time by the
method of magnetic retention and to close the movement of plasma
particles in a tokamak or stellarator. The
plasma density used here is relatively low n » 5.1017 cm-3, but the retention
time tE tries to reach the order of a few seconds.
Both of these methods will be discussed in more detail below :
Possibilities
of technical implementation of controlled thermonuclear fusion
Attempts to carry out a controlled thermonuclear reaction proceed
in two fundamentally different ways according to the method
of maintaining the required temperature and density of
the reaction plasma cloud (some alternative methods will be
discussed below) :
¨ Inertial fusion - inertial retention of plasma ,
in which rapid local heating of a
small volume of nuclear fuel results in the formation of very hot
and dense plasma and thermonuclear fusion on a
small scale, before this fuel can be explode to the environment
by thermal motion. The plasma is not held by any external force
field, but simply by its own mechanical inertia. For a short time
(approx. 1 ns) they are maintained by the "inertial
resistance" of the mass to acceleration, by its inertia the
plasma remains compressed in its original place for a certain
sufficient time. The principle of this method (whose not very apt name arose from the use of inertia
and the law of action and reaction) is
shown in Fig.1.3.8 left. A small capsule of nuclear fuel,
containing several milligrams of D+T, is irradiated from several
directions simultaneously by high-power pulses of radiation from lasers
or particle beams (Phase A). The absorption of this radiation
leads to a sudden heating of the surface
layer of the capsule (so-called ablator), which
evaporates rapidly and expands into space. Due to the law of
action and reaction, this rapid expansion of the evaporated
ablation layer results in rapid compression,
implosion, of the inner part of the capsule D+T - the effect of a
"spherical rocket engine" - Phase B. The inside of the
capsule should be briefly compressed to a central density of
about 200g/cm3, with a simultaneous sharp rise in temperature (adiabatic effect).
If the temperature in the middle exceeds the ignition
temperature, the fuel will "ignite" thermonuclearly and
the wave of thermonuclear "burning" will rapidly spread
to other parts of the fuel. In a strongly compressed and adiabatically
heated plasma inside the capsule, thermonuclear fusion
of D and T can occur - a kind of "thermonuclear
micro-explosion" (Phase C), in which up to about
30% of the amount of D+T mixture can fuse to 4He and flying-out
neutrons no.
Helium nuclei and neutrons fly out with high kinetic energy, for
a total of 17.6 MeV/1 fusion.
It is very
important to ensure instantaneous perfectly isotropic
homogeneous irradiation, so that the capsule is compressed
as symmetrically as possible. This is not easy to
achieve with several independent lasers, so a single default
laser is used, whose light is split into multiple beams,
amplified and directed at the target. Neodymium lasers
are mainly used in the current experiments for primary
high-performance irradiation of inertial fusion fuel with a large
number of beams (often more than 100). It shines at a wavelength
of 1.054 mm (infrared region), in the final phase the conversion
to the 3rd harmonic 0.351 mm (ultraviolet region) is usually performed. A short
power pulse is obtained by powering from a charged capacitor
batery. The priming laser beam is divided into a plurality of
beams (channels) which are passed through laser
amplifiers consisting of a series of glass plates
with neodymium admixture. These plates are irradiated with xenon
lamps that excite neodymium. As the laser beam passes, stimulated
deexcitation occurs, amplifying the laser beam. The laser beams
thus amplified are then guided from many different directions
into a reaction chamber, in the center of which they converge
into a capsule with nuclear fuel.
Recently, attempts to make an additional "rapid
ignition" (Fast Ignition) thermonuclear
fusion: compressed implosion plasma stage (Stage B obr.1.3.8
left) is subsequently irradiated with radiation from a short
burst of high power laser beam to a diameter of concentrated »1 mm, where the
intensity is approx. 1017 W/cm2. The absorbed energy strongly increases the temperature
in the center, which can lead to a more efficient ignition of the
thermonuclear fusion. This can save energy for the primary
irradiation of the capsule.
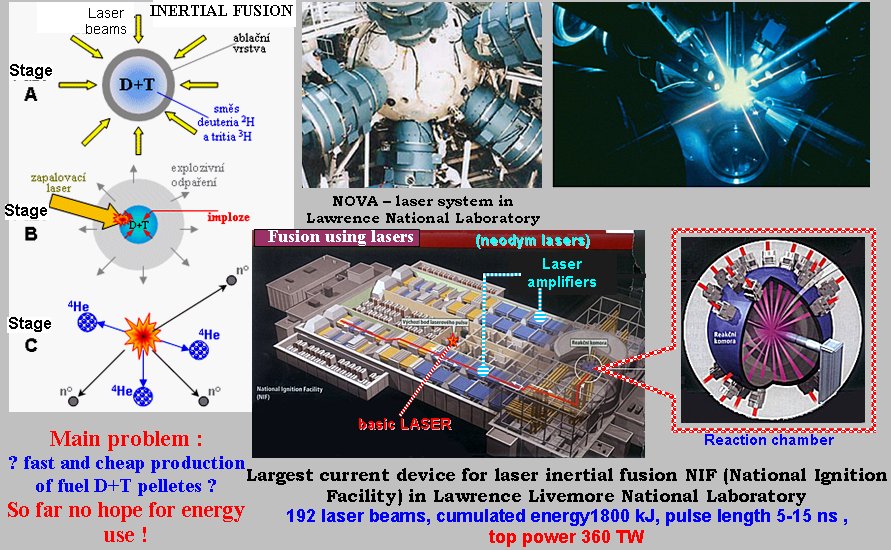
On the ablator material specific requirements
are placed, especially high ablation rate and good absorption of
radiation from lasers, low reflectivity and high opacity. Plastic
materials (possibly doped with germanium), beryllium (possibly with an
admixture of copper), high-density carbon (prepared by the method of production of artificial
diamonds) are tested.
The method of direct energy supply
to the nuclear fuel target according to Fig.1.3.8 on the left is
referred to as "directly driven inertial fusion".
In order to achieve the required pressure and temperature in the
center, high demands are placed on the homogeneity, symmetry and
isotropy of energy absorption from the external rays. Therefore,
experiments are being made with so-called "indirectly
driven inertial fusion", where the capsule with the
fuel is placed inside a suitable package (made of a material with
a high atomic number, eg gold) - in a hollow tube *), the inner
walls of which are irradiated with laser beams through openings
at the end; sources converted to soft X-radiation in thermal
equilibrium, acting isotropically a capsule with fuel.
*) This reaction vessel is sometimes called
"hohlraum" (from german cavity). This
method is probably only a makeshift laboratory solution that will
probably not be applied when other attempts energy utilization
inertial thermonuclear fusion ...
A
thermonuclear reactor based on this principle of inertial
fusion ICF (Inertial Confinement Fusion) would
operate in a fast pulse mode, where the focus of the laser beams
would be in small capsules of nuclear fuel (D+T) were thrown in quick
succession, and synchronous triggering of lasers would
induce thermonuclear fusion in each capsule. Furthermore, it
would be necessary to solve the removal of the released energy
from the reaction space (cooling technique); the main part of the energy carried by neutrons would
be drawn by cooling the envelope (blanket) of the
reaction chamber, made of neutron absorbing material (beryllium or lithium - similar to tokamaks, see below).
Currently, the
two largest experimental devices for laser inertial fusion are
operating :
- NIF (National Ignition Facility) at
Lawrence Livemore National Laboratory in California, USA,
completed in 2010. It consists of 192 laser channels.
- LMJ (Laser MegaJoule ) in Le Barp
near Bordeaux in France, completed in 2006, has 256 laser beams.
Both of these devices achieve a cumulative energy of laser beams
of 1800 kJ with a pulse length of 5-15 ns and a peak power of 360
TW.
We are still
very far from the creation of a thermonuclear power plant, that
would be able in inertial fusion microreactions to release energy
covering its entire necessary energy input and produce additional
energy. Inertial fusion will probably never be used in
terrestrial energetics. Much further in this direction is the
method of magnetic plasma containment using tokamaks, described
below. However, the future use of inertial thermonuclear fusion
of deuterium and helium-3 *) to propulsion of interstellar
rockets is being considered - cf. below "Nuclear
Propulsion of Space Rockets"..?..
*) Unstable tritium with a half-life of 12 years does not occur
in space. However, helium-3 is found on some planets from where
it could in principle be extracted, along with deuterium.

Fig.1.3.8. Two basic methods of controlled thermonuclear fusion.
Left : Simplified principle of
inertial fusion and the course of thermonuclear micro-explosion.
Right : Simplified tokamak
schematic diagram.
¨ Magnetic holding and closing of
high-temperature plasma ,
which is performed in so-called tokamaks
(Fig.1.3.8 on the right). The tokamak *)
consists of a toroidal working chamber placed in
a strong magnetic field inside a coil wound around the chamber in
a toroidal arrangement. The lines of force of this toroidal
magnetic field run along the long circumference of the toroid.
This whole toroidal system is further "threaded" on the
ferromagnetic core of the "transformer", the primary
winding of which, wound on the core in the axis of the toroidal
system, is supplied with alternating current. Single
"secondary circuit thread" (as if
connected in "short circuit") of
this transformer forms a ring of high temperature plasma inside
the working toroidal chamber. Plasma has good electrical
conductivity, so a strong electrical current it induces in it
(for larger devices even several million amperes). This electric
current both causes induction heating of the plasma
to a very high temperature (approximately 10 million degrees),
and also creates a magnetic field in the so-called poloidal
direction with lines of force directed along the shorter
circumference of the tube. The entire tube is further surrounded
by external coils of the poloidal field, which shape the plasma
and contribute to the equilibrium of forces in the plasma. By
combining the stronger toroidal and weaker poloidal components of
the magnetic field, "helical" slowly twisting lines of
force of the resulting magnetic field are created, within which
plasma particles can move along closed paths. This result in the magnetic
closure of charged plasma particles into the center of
the toroidal working tube.
*) The word "tokamak" originated
as an abbreviation of the name "toroidalnaja
kamera s magnitnimi katuškami"
- toroidal chamber with magnetic coils. The device was
developed as early as 1951 by a team led by A.O.Lavrentev,
A.D.Sacharov, I.E.Tamm and L.I.Arcimovic in Kurchatov's nuclear
laboratories in the USSR.
These two mutually perpendicular
magnetic fields - toroidal and poloidal - form a kind of "magnetic
vessel" inside the toroidal plasma chamber or a
"trap" in which Lorentz forces acting on moving
electrically charged plasma particles (D and T cores) hold the
resulting plasma in the toroid axis and do not allow immediate
escape of the particles by thermal motion to the chamber walls
*). The plasma seems to "levitate" in the tube space,
and during the working cycle, the hot plasma is kept at a
sufficient distance from the walls of the tube (the temperature of the chamber walls should not exceed
1000 °C) **). If this time of
magnetic retention of the plasma, heated to a
sufficiently high temperature, is sufficiently
long relative to its density, conditions for the
nuclear fusion of D and T nuclei can occur in
the tokamak chamber (in accordance with the
above Lawson criterion).
*) The compression of a discharge in the
plasma by magnetic forces into a thin strand is referred to as a
"pinch-effect" ( pinch - pressing,
clamping, constriction ). It is commonly observed in spark
discharge, lightning, solar protuberances.
**) In some tokamaks, a poloidal limiter is used - an
annular aperture defining the cross-section of the plasma to
reduce the thermal load on the tube wall.
Equilibrium of plasma
and magnetic field in a tokamak tube
The basic mechanism of magnetic plasma retention is magnetohydrodynamic
phenomena in an ionized substance, in which the pressure
gradient is given by the Lorentz force, determined by the product
of the (vector) electric current density and the magnetic
induction. At equilibrium, the pressure gradient in the plasma is
then perpendicular to the lines of force of the magnetic field
and also perpendicular to the direction in which the electric
current flows. Constant pressure surfaces, determined by the
directions of the electric current and the magnetic field, are
created in the plasma. Under simplified conditions, the
equilibrium arrangement of the magnetic field and plasma can be
described by electrodynamics of a continuum with free charge
carriers (such a description was made in 1958-66 by H.Grad, H
Rubin and V.D.Šafranov in radial coordinates with toroidal
symmetry). In fact, plasma drifts and magnetic field expansion
occur in the toroidal tube, leading to an additional vertical
toroidal electric current (described in 1962 by D.Pfirsch and
A.Schlüter). In practice, the situation is much more
complicated, the turbulence of heat transfer and particle in
plasma is applied. ....
Stellarator
A separate variant of tokamak are so-called stellarators
(Stellar generator - "star generator",
developed by group of L.Spitzer in the USA), which do not have a
central primary winding and in which all components of the
magnetic field are generated by intricately configured external
coils. Stellarators probably not be used as energy thermonuclear
reactors in future, but they are of considerable experimental
importance. They make it possible to model in more detail the
different shapes and courses of magnetic fields and their
influence on the behavior of high-temperature plasma in terms of
thermonuclear reactions.
Sferomak, Dynamak
Other methods using special magnetohydrodynamic phenomena
are also tested due to currents generated inside the plasma
itself (these are phenomena similar to the
coronary loops of the Sun or relativistic jets from accretion
disks). It is tested in experimental
facilities called Spheromak. ......... The combination of
electric currents inside the plasma and their magnetic fields
results in closed magnetic field lines that can maintain the
shape of the plasma ring without the need for or with the
minimization of strong external electromagnets. This would allow
the construction of simpler and less expensive fusion devices
than tokamaks. This method is being developed in the laboratories
of the University of Washington, and the experimental instrument
has been given the working name Dynamak .... .
Tokamak duty cycle
Tokamak operates in cyclic pulse
mode. At the beginning of the cycle, "fuel", D+T
gas with a density of about 1015-18 particles/cm3, is filled into the evacuated toroidal chamber. Then an
electrical voltage is applied to the primary winding of the
"transformer" from a very powerful ("hard")
source. With an induced current of many thousands to millions of
amperes, the plasma is heated rapidly to about
107
degrees, while being maintained in the toroid axis by the
magnetic field.
Induction heating - ohmic
If an electrical voltage Uprim is applied to the primary winding of a tokamak, an
electric current Iprim begins to flow, which changes with time according to
the exponential dependence Iprim(t) = (Uprim/Rprim).(1 - e-t.Rprim/Lprim), where Rprim is the total active resistance of the primary circuit
(sum of the resistance of the turns of the primary winding and
the internal resistance of the source) and Lprim is proper primary winding inductance. This rapidly
increasing current through the primary winding generates a
rapidly increasing magnetic field B(t) in the
direction of the toroid. According to Faraday's law of
electromagnetic induction, an electromotive force
will be induced inside the tube along the toroid Utor = -dF/dt = -S.dB/dt,
where F(t)
= B.S = Lprim.Iprim is the magnetic flux through the surface S of
the toroid. In the gas charge of the toroid, due to electric
forces, free electrons *) begin to accelerate, which ionize the
gas with shocks (avalanche process). A electrical
discharge will be gerated and a current Iplasm = Utor/Rplasm will flow trough
the plasma, where Rplasm is an electrical (ohmic) resistance of the plasma. The
ionized gas plasma in a toroidal-tube starts quickly heated by
the Joule heat of power P = Rplasm .Iplasm2. It is an ohmic or resistance
heating with induced current.
*) There is a small amount of electrons and
ions in each gas due to natural radiation. When using a mixture
of D + T, a large amount of electrons and ions is formed due to
the radioactivity of tritium. In experiments with a neutral gas
(eg pure H), it is appropriate to initiate the initial weak
ionization by electron injection.
With a sufficiently powerful supply
of the primary winding, the plasma in the toroidal tube is
inductively heated to a temperature of approx. 107 degrees within a few
milliseconds. With increasing temperature T increases the
degree of ionization and decreases the electrical resistance of
plasma (Rplasm ~ T-3/2) - inductive heating is no longer effective. At this
point, for the next required temperature increase, additional
heating - non-inductive - must start from
external sources :
Additional hybrid
heating of plasma -
non-inductive
Additional non-inductive plasma heating can be performed in two
basic ways :
¨ Electromagnetic waves
of high frequencies - microwaves, which are absorbed in plasma,
vibrates charged particles and its energy is converted into
thermal motion of electrons and ions in the plasma. Frequencies
from about 20 MHz to many GHz are used. The absorption of
electromagnetic waves are most effective, when their frequency resonates
with some natural oscillations in the plasma. One such
oscillation is the so-called cyclotron rotation
particles (ions and electrons) along magnetic field lines with
(Larmor) frequency given by the charge of the particle, its mass
and the intensity of the magnetic field. In a typical tokamak
with magnetic field of about 4-8 T, deuterium ions have a
cyclotron frequency in the range of about 20-60MHz. Plasma
heating using microwaves with a frequency of ion rotation in a
magnetic field is called ICRH (Ion Cyclotron
Resonance Heating). Electrons, which are about 4,000 times
lighter than deuterium ions, have much higher cyclotron
frequencies - of the order of 100 GHz. The method of heating
plasma using microwaves with this high frequency of electron
rotation in a magnetic field is called ECRH (Electron
Cyclotron Resonance Heating). Particulalarly effective is
heating with microwaves of about 70-200 GHz excited in gyrotrons
(their principle is described in §1.5,
passage "High frequency generators"). The so-called hybrid
frequencies arising from the combination (geometric average)
of electronic and ionic cyclotron frequencies are also used.
Their values lie between the ionic and electron cyclotron
frequencies; the use of microwaves of the so-called lower
hybrid frequency for non-inductive excitation of toroidal
current in plasma is mentioned below. Such radio frequency waves
could provide both additional heating of the plasma and the
generation of an electric current in the plasma to generate a
poloidal magnetic field.
¨ By injecting neutral accelerated particles
- atoms which transfer their kinetic energy by collisions
with plasma particles and thus increase the temperature. The
particles must be neutral so that the strong magnetic field in
the tube "lets" them into the interior of the plasma.
Generating intense neutral beams of fast atoms is not easy. We
cannot accelerate neutral particles, so it is necessary to first
accelerate charged ions (hydrogen, deuterium, or
tritium) in a high voltage field to an energy of about 100keV -
1MeV and then neutralize these accelerated ions by
passing through a gaseous medium. From the point of view of
neutralization, it seems more advantageous to accelerate the
negative ions, which are more easily neutralized when passing
through a gaseous medium (by strip- interaction by
electron detachment). The use of accelerated heavy ions
also seems promising (eg cesium ions were tested).
After reaching
the ignition thermonuclear temperature (min. 108 °C), part of the
energy released by the plasma fusion reactions it further heats
the plasma - "self-heating" occurs and
thermonuclear fusion can continue "alone". The fusion
reaction is able to maintain the required temperature if the
power released by the fusion covers self-heating and energy
losses. When this "burning plasma" is reached, the
external heating can be reduced or switched off.
If the primary
winding of the tokamak "transformer" is connected to a
DC voltage source, the magnetic flux in the core soon saturates,
the electromagnetic induction disappears and the plasma current
ceases - the poloidal field disappears and the plasma begins to
escape from the center of the tube towards the walls. This
problem can be solved in two ways :
1. By quickly reversing the polarity of the
power supply of the primary winding, which restores the induction
in the opposite direction.
2. By applying high-frequency electromagnetic
waves of a frequency of several GHz (approx. 2-5 GHz) in the
tangential direction to the plasma. With proper synchronization,
the electrons will be accelerated - "dragged" - at the
wavefront (similar to the linear accelerator - LINAC), creating
an electric current in the toroidal direction, generating the
required poloidal magnetic field. This method of non-inductively
exciting the plasma current by microwave radiation is sometimes
referred to as LHCD (Lower Hybrid Current Drive); the
name is related to the use of the so-called lower hybrid
frequency lying between the above-mentioned ionic and
electron cyclotron frequencies (ICRH, ECRH - is their geometric
average). At this frequency, a component of the electric field
parallel to the magnetic field will accelerate the electrons
moving along the field lines.
In addition,
the so-called bootstrap curent can be applied : Due to
the variable plasma density, a current in the toroidal direction
is automatically generated, which can contribute to the formation
of a poloidal field. It is an internal process that takes place
spontaneously without external excitation.
After
completion of a given cycle of fusion reaction, the particles
remaining after reaction (helium, remaining D and T, impurities
formed by the action of plasma to the tube walls) are pumped
out by means of a divertor, located
around the circumference at the bottom of the tube. The device is
then ready for the next cycle. In the case of continuous
operation, it would be necessary to ensure the continuous removal
of the reaction products.
Divertor - "plasma
cleaner" - consists of collecting plates that capture helium
atoms, unburned hydrogen and impurities released from the walls
of the tube (or penetrating leaks into the vacuum tube). The
basic toroidal magnetic field is modified so that the magnetic
field lines at the outer edge of the plasma ring direct the
plasma particles into the region of the divertor. The ions are
captured here on the collecting plates and recombined into
neutral atoms. The gas generated in this way is sucked out of the
divertor area by powerful pumps and discharged (it is a kind of "exhaust gas" from the
reactor). The effluent atoms of the
unburned hydrogen fuel are separated from the helium
"ash" and impurities and can be reused for fusion. The
divertor is highly thermally stressed and must be intensively
cooled. In large tokamaks with a suitably shaped magnetic field,
thermal and radiation stress can be reduced due to the relatively
long path of the "waste" plasma particles from the edge
of the plasma ring to the divertor region; the plasma simply
cools to such an extent that electrons and ions recombine into
neutral atoms before they reach the surface of the collecting
plates.
Part of the
fusion of the released energy heats the walls of the tube and the
divertor (drained by coolant), most are carried away by
high-energy neutrons, which are not captured by the magnetic
field or the tube wall, but by the reactor envelope - blanket
- water-cooled material containing beryllium. Instead of
beryllium, the use of lithium 6,7Li
seems promising here, which would not only be a neutron
absorber, but by neutron absorption, lithium would be converted
to tritium *) (as discussed above), which would
make it possible to obtain and burn the most difficult to obtain
fuel component (and in addition
radioactive) - tritium T º 3H1 in a closed circuit (Fig.1.3.8 right).
*) A sufficient amount of tritium here can be obtained by a
cascade of two reactions:
1. The neutron hits the lithium 7nucleus,
creating a tritium ion and a neutron (endothermic reaction);
2. This
secondary neutron strikes the nucleus of the lithium isotope 6
and produces a second tritium ion.
All this tritium needs to be collected with high efficiency and
introduced into the plasma in the reaction tube. A suitable
material for a tokamak blanket could be a eutectic LiPb
lithium-lead alloy.
From the point of view of energy
utilization, the energy yield Q is important,
which is the ratio of energy obtained from fusion to energy that
needs to be supplied to excite magnetic fields and create high
temperature plasma :
Q = [thermonuclear
power] / [external
power to create and maintain plasma] .
In previous smaller experimental tokamaks, if they
achieved fusion at all, the energy yield was very low, Q
<0.01. Newer large devices already achieve a yield of about
0.6 (JET). For practical use in energy, it is necessary to
significantly exceed the profitability limit - Q
>> 1; the planned ITER should have a Q » 10.
Design development of
tokamaks
The construction arrangement of tokamaks has undergone a number
of technical modifications and improvements since their
inception. E.g. the cross-section of the plasma toroidal tube is
no longer circular or elliptical, but a cross-section of a shape
similar to the letter "D", with a straight part adjacent to the central
electromagnet, has proved to be more advantageous; this shape
better copies the actual configuration of the constant magnetic
flux surfaces in the poloidal cross-section of the tube, which is
established as a result of magnetohydrodynamic processes. In the
lower part of the chamber, the collector plates of the divertor
are arranged around the perimeter for the capture and removal of
reaction products and unwanted impurities. The former manual
control of tokamaks has been replaced by fully electronic
regulation all phases of the cycle, using computer
control and simulations.
We are also working on the
possibility of replacing the current pulse mode with a continuous
mode: deuterium and tritium *) would be fed into the
reaction tube, where the high-temperature plasma would be
maintained permanently, the helium ions formed would be separated
out. However, superconducting electromagnets are
a prerequisite for continuous operation, as conventional copper
coils make it possible to maintain the fusion reaction for only a
few seconds without overheating. The possibilities of using
reactions other than D+T are also being considered, which,
however, would usually require an even higher plasma temperature (discussed below).
*) Refueling hydrogen inside the hot plasma
ring in a continuous mode encounters technical difficulties.
During normal filling of atoms, due to the high temperatures in
the tube, the atoms are rapidly ionized and a strong magnetic
field prevents them for the necessary penetrating into the center
of the tube. Hydrogen fuel must therefore be injected at
a high speed of a few km/s, either in the form of gas,
or small capsules of frozen hydrogen (which can reach the central
region of the hot plasma before it evaporates).
High technical
demands are placed on the wall material of the
working toroidal tube :
¨ High mechanical
strength ;
¨ High thermal
resistance (>1000 °C), including resistance to rapid
temperature changes, good thermal conductivity and cooling
capability ;
¨ High radiation
resistance, especially to intense neutron flux (energy 14 MeV), as well as low
effective cross section for nuclear reactions causing activation
(formation of radioactive elements in the material) ;
¨ Gases should not
be released from the inner wall material, which could break the
vacuum inside the tube, contaminate the plasma and violate the
conditions for proper fusion.
Special composite materials based on
tungsten, carbon, beryllium are used, with a special coating of
the inner wall, which comes into direct contact with hot plasma.
Alloys of tungsten, tantalum, vanadium and chromium with special
quaternary crystallization, which show high temperature and
radiation resistance, are also tested. The inner wall can be
covered with graphite, or coated with beryllium.
The largest tokamak to date is the JET
device (Joint European Torus),
built in collaboration with several european countries in
Abingdon (Oxfordshire), UK, with a main
toroidal tube radius of 2.96 m. It is capable of producing a
thermonuclear power of 4 MW for 4 seconds, with efficiency Q»0.62.
Large Tokamak ITER
Currently, a project of a new and improved considerably larger
tokamak ITER (International Thermonuclear
Experimental Reactor *) is
gradualy realized, in partnership with the European Union and
several economically strongest countries in the world (being built in the south of France), which will have more than twice the diameter of the
toriod chamber (6.2 m). More than 20 powerful microwave
generators - gyrotrons (described
in §1.5, passage "High frequency generators") will be placed around the
perimeter of the working toroidal chamber, providing additional
non-inductive heating of deuterium-tritium plasma.
*) One of the Latin meanings of the word iter
is the path - we believe that it will
be the right path for the technological mastery
of thermonuclear energy..!..
 |
 |
All electromagnets will be here superconducting (physical principles of superconducting magnets are briefly discussed in §1.5, section "Electromagnets in accelerators", section "Superconducting electromagnets"), which will significantly reduce the consumption of electrical energy to excite the magnetic fields. This thermonuclear reactor will therefore already have a positive energy yield - will be able to release more energy than the energy supplied (Q » 10). There will also be examined by the above production technology of tritium from lithium (fusion reaction with neutrons, as described above) in a closed cycle.
Difficulties and perspectives of
thermonuclear fusion
In the 60.-70. years when many successes were achieved in
improving the tokamaks, there was general optimism.
Most nuclear physicists were convinced that thermonuclear fusion
would be successfully mastered and technically exploited by the
end of the 20th century.
I remember as a student of the Faculty of
Mathematics and Physics in the 1970s that our professors and we
students were then convinced that after 2000, "obsolete
fission nuclear reactors" will be shut down and energy in
power plants will be obtained in thermonuclear fusion reactors...
However, further experiments, in an
effort to maintain a sufficiently hot and dense plasma for
thermonuclear fusion for a longer period of time, began to
encounter serious difficulties. One of the main
problems is plasma instability - its
oscillations and turbulence, leading to too high
heat diffusion in the plasma and thus to large energy
losses, shortening the retention time of thermal energy inside
the plasma.
Simply put, plasma "has no desire to submit to us": the
hotter the plasma we create and the harder we compress it, the
more it "hinders" our efforts to keep it; finds a way
to "burst" to the sides ...
The time of
holding the thermal energy inside the plasma can be increased in
basically two ways :
Greenwald's plasma density
limit in tokamak
In tokamak experiments, it is observed that the plasma becomes
uncontrolled - it expands beyond the magnetic fields that keep it
in the tokamak tube - when its density rises above a certain
limit. Based on a series of experiments on several tokamaks, M.
Greenwald in 1988 set an upper limit of plasma density nGw , above which it can
no longer be magnetically held in a tokamak tube: nGw [1020m-3] = Ip /p.a2 , where Ip is the plasma current
[MA], a is the smaller tube radius [m].
This Greenwald limit
represents an unfavorable circumstance caused by a
number of factors - radiation losses, discontinuities in the
peripheral parts of the plasma, differences between power input
and radiation transport processes, released impurities (such as carbon and tungsten) into
the tokamak plasma from divertors and limiters, higher thermal
conductivity of dense plasmas (heat losses
may exceed energy input - thermal quenching may occur).
Overall, the Greenwald plasma
density limit is due to the same effects that affect the energy
balance in the plasma environment in the toroidal tube -
mentioned above. Additional hybrid plasma heating in newer
tokamaks may increase the Gw density limit. Some
recent experiments further show that plasma can maintain a stable
higher density, if is increasing the performance of the
fusion reaction. This could increase the achievable power of
large tokamaks..?..
Increase performance
and retention time. L-mode and H-mode of the tokamak
In an effort to increase the power P in the tokamaks, the
retention time tE (roughly as tE ~ P-1/2) began to decrease due to greater turbulence. However,
in experiments with medium-sized tokamaks, it was observed that
increasing the power at a certain value significantly suppressed
the turbulence in the plasma. This is a beneficial
phenomenon which increases the efficiency of magnetic
confinement, and retention time tE. This mode has been called H-mode (high
confinment mode) and the normal mode (preceding this mode)
is called L-mode (low confinment mode).
...
In addition to the spontaneous onset
of H-mode, other methods of increased plasma retention in the
tokamak are being tested. Increased power and hold time can also
be achieved by fine-tuning the current and electrical
profile of the pulse (in feedback), which creates internal
transport barriers to plasma fluctuations. Also, specific hybrid
heating scenarios have a stabilizing effect on plasma by
reducing transport processes leading to losses and turbulence.
Serious technical problems that can stand in
the way of energy use of thermonuclear fusion are associated with
thermal and radiation stress of the walls of the
toroidal tokamak tube (especially very
strong neutron flux) and the surrounding
blanket, their cooling and heat transfer to the electric
generator - unfortunately it won't work without steam
yet *)....
*) The theoretical exception could be special neutron-free
fusion reactions, where the fusion energy is carried
away by charged particles, which could be - at least in
principle - used for direct conversion to electrical
energy, without the need to convert via heat - is
briefly discussed below in the passage "Neutron-free fusion".
The custom
ignition of a thermonuclear reaction may be the easier part of
the whole problem of fusion energy utilization ! We do not yet
have materials capable of withstanding high temperatures and
intense bombardment by high-energy nuclear particles (subatomic stress). New
materials with significantly improved thermal,
mechanical and radiation properties will need to bedeveloped, and
a new technical solution for the liquid blanket is being
considered, where the solid materials of the blank
enclosing the fusion chamber would be replaced by liquid
materials (lithium-containing), which would circulate and thus transfer heat well,
effectively cool the chamber and the necessary tritium could be
separated from them.
The magnetic field in a tokamak must be
generated in superconducting electromagnets (the physical principles of superconducting magnets are
briefly discussed in §1.5, section "Electromagnets in accelerators", section "Superconducting
electromagnets"), so that the energy for its generation does not exceed
the energy obtained during fusion. Appropriate cryogenic systems,
operating at temperatures close to absolute zero, are located in
close proximity to plasma heated to hundreds of millions of
degrees! In addition, neutrons adversely affect the
superconducting materials of the coils. How to insulate
heat-radiation these lowest and highest temperatures what can we
imagine?
A major problem with D-T fusion may
be the recovery of tritium 3H, which, unlike deuterium, is very rare in nature (we cannot yet create the conditions for the fusion of
deuterium itself or the other reactions mentioned below). We have presented nuclear reactions that allow a
thermonuclear reactor itself to "produce" the necessary
tritium - D-T (-Li) cycle. Inside the envelope
(blanket) of the reaction tube or vessel, there will be channels
with lithium, which will capture neutrons and form helium and the
necessary tritium, drained channels and after separation injected
back into the reaction space (Fig.1.3.8
right). In each fusion reaction, one
tritium nucleus is consumed and one neutron is formed. Each
neutron flying out of the reaction tube must produce at least one
tritium nucleus - otherwise the system will soon run into a tritium
deficit (it will consume more tritium than it will
produce) and D+T fusion will no longer be maintained. This can
only be achieved through a series of two reactions:
In the first reaction, the neutron hits a 7Li nucleus to form both a tritium and a flying neutron (this is a slightly endothermic reaction). The released secondary neutron during its flight in
the blanket then hits the 6Li nucleus and produces a second tritium nucleus (+ helium; it is an exothermic reaction). All the tritium formed in this way must be collected
with almost 100% efficiency and reintroduced into the reaction.
Together with the fact that not all neutrons will participate in
the required reactions (some of them always
escape or be swallowed differently) , this
is a difficult problem to solve ...
Experiments on previous tokamaks,
especially on JET, have already contributed to solving many
problems. Much is expected from the forthcoming ITER
project , which perhaps could be completed around 2040
in southern France and the experimental stage could take min. 20
years. It should be followed by the DEMO tokamak and then the
energy thermonuclear reactors. Therefore, if there is no happy
turnaround (finding new advantageous technical solutions), the
beginning of the energy use of thermonuclear fusion can be
expected in the best case only in the
second half of the 21st century.
The second basic pathway studied, inertial
fusion, is still at an even earlier stage of
development than tokamaks. Instead of the necessary fast sequence
of fusions, experiments are currently being carried out with
individual laboriously and expensively prepared D+T fuel
capsules. Until the routine production of a large number of cheap
fuel capsules is mastered, inertial fusion cannot compete with
tokamaks.
Alternative options for nuclear
fusion
Electrical
acceleration and plasma retention - fusors
In addition to heating nuclear fuel to a very high temperature,
light nuclei can be accelerated to effect their fusion using an electric
field. A certain advantage of this method is that, in
addition to accelerating the nuclei, it directs their movement,
so that instead of accidental movement during thermal collisions,
there is a targeted collision of the nuclei "against each
other". When directing the chaotic movement of fuel
particles, we do not have to reach such high energies ~
temperatures as with tokamaks, smaller and simpler devices will
suffice. A device that uses an electric field to accelerate and
collisions ions under conditions suitable for nuclear fusion is
called a fusor. The first prototype fusor was
designed in 1964-67 by P.T.Fransworth and R.L.Hirsh. The fusor is
basically a large vacuum tube with two spherical concentric
electrodes made of wire mesh, the cathode is inside the anode.
Fill the flask with a dilute fuel mixture (D+T). The voltage
between the outer and inner electrodes causes ionization and then
accelerates and at the same time directs the movement of the fuel
ions towards the center. Accelerated ions fly through the cathode
wire mesh on all sides and collide at the
center, which can cause nuclear fusion in a non-thermal
manner. There are the inertial electrostatic plasma
retention (IEC - inertial
electrostatic confinement).
The internal electrode (cathode)
causes certain problems with fusors, on the wires of which a
large part of the ions terminate, and due to bombardment by these
fast particles, the electrode material degrades rapidly. An
improved variant of fusors is called polywell (the name originated as an abbreviation of the Greek polyhedron
= polyhedron and English well = well, pit - here a potential
pit). It does not have an internal
metal electrode, instead a strong magnetic field is applied by
means of superconducting coils arranged in a polyhedron, most
often 6 coils arranged as cube walls. Electron nozzles are
located in the axes of the coils, high voltage is applied between
the coils and the nozzles. Electrons fly out of the electron
nozzles towards the center, where they are captured by the
magnetic field. The resulting cloud of electrons with a negative
charge in the center forms a kind of "virtual cathode"
that attracts positive ions. These cores accelerate in the
electric field between the coils and the virtual cathode and
collide in the middle, with the possibility of mutual fusion.
So far, it has not been possible to
design the fusor device so that it supplies more energy than it
consumes by nuclear fusion, or it has at least partially
approached this goal. However, the fusors were used as suitable
laboratories neutron sources (cf. §1.5, section "Neutron generators").
Muon catalysis of
nuclear fusion
A certain physical way to reduce the repulsive electrical force
of nuclei and thus allow fusion at lower temperatures (with the exaggeration of "cold fusion"), perhaps the use of muons m-
(§1.5, section "Elementary particles and their
properties"). Negative muons are no different from electrons except
for mass and instability, so they can replace them in the
electron shells of atoms. This creates so-called mesoatoms (muons were formerly called mi-mesons). The hydrogen mesoatom represents a bound system (p+ m-) in which an electron in a hydrogen atom (deuterium,
tritium) is replaced by a muon; on the outside it is electrically
neutral. The muon is 207-x heavier than an
electron, so it orbits in a much closer orbit around the
deuterium nucleus - the better it shields the electrostatic
repulsion from another hydrogen nucleus. The size of such
mesoatoms will be much smaller than that of atoms (1000 times smaller than a hydrogen atom) and the nuclei may be closer to each other, which would
lead (in co-production with the tunneling phenomenon) to an
increased probability of merging two of their nuclei even at lower
temperatures. After fusion, the muon is released and can
enter another mesoatom and catalyze further
reactions.
If muons were stable particles, or
at least with a lifespan of the order of milliseconds, muon
catalysis would perhaps be a promising option for low-temperature
nuclear fusion. However, the muon decays in a few microseconds (half-life 2.2.10-6 s), so in its short life it may be sufficient to catalyze
only a few fusion reactions (roughly 5). In addition, some of the
muons may be trapped on the helium nuclei formed during the
synthesis and thus be lost from the catalytic circulation. Muons
have to be produced in a relatively complicated and energy
consumed way in an accelerator. In this situation, the energy
required to produce muons would be many times higher than the
energy extracted during fusion. The possibility of energy
utilization of muon catalysis of nuclear fusion is not
yet feasible in practice !
(no) Possibility of
cold nuclear fusion?
Occasionally there is speculation about the possibility of "cold"
fusion at or near normal room temperatures. In
1989, chemists M.Fleichsman and S.Pons (University
of Utah) described an experiment with heavy
water electrolysis using palladium electrodes, which allegedly
involved nuclear fusion of deuterium. The fusion reaction should
perhaps have been catalyzed in the palladium crystal lattice.
They inferred the fusion reaction from a not very convincing calorimetric
measurement; their electrochemical laboratory did not have
the appropriate detection technique to measure gamma and neutron
radiation, which would be a key indicator of fusion. No one else
has succeeded in repeating this experiment positively, so
practically all experts are absolutely skeptical about it...
Nuclear physics convincingly shows
that cold nuclear fusion is not possible, as it
is prevented by large electrical repulsive forces
between positively charged nuclei. No chemical or electrochemical
methods in any substance can achieve a sufficient proximity of
the nuclei to a distance of the order of 10-13
cm to apply a strong interaction,
which only can lead to fusion.
*) Hypothetical possibility of cold
fusion in space ?
When speculating about the extremely long-term development of the
open universe (§5.6 "The
future of the universe. The arrow of time. Dark matter. Dark
energy.", the passage
"Open universe" in the monograph "Gravity, black holes and
space-time physics"), in the time horizon >>10100 years , the quantum
tunneling effect could hypothetically be applied, which
with a small probability would help to overcome the strong
electrical repulsive barrier between atomic nuclei. In rare
cases, spontaneous cold fusion of lighter nuclei into
heavier nuclei would occur. This exotic process will forever
remain only hypothetical, it will never be possible to verify it
experimentally ...
Instead of "cold fusion", however, according
to some opinions, it could hypothetically be possible to realize
other reactions, which on a smaller scale could somewhat resemble
cold fusion in external manifestations: low-energy
nuclear reactions (LENR) taking place primarily with the
help of the weak interaction. A certain possibility is
to produce neutrons (using very
strong alternating electromagnetic fields to achieve the fusion
of an electron with a proton to form a neutron), which would be absorbed by suitable atomic nuclei.
These nuclei would become beta-radioactive and the energy of the
emitted particles could be harnessed..?..
There is a bit
of a similar information situation around cold
fusion as with perpetuum mobile (or in UFO ).
From time to time, sensational news flashes about the success of
fraudsters or amateur researchers -
"do-it-yourselfers". It usually "fizzle out"
soon, but soon others appear. Experts do not usually deal with this, on the
refutation of errors or fraud is written only marginally in
professional magazines, they do not reach the lay public. Many
people are thus convinced of the possibilities and real
perspective of cold fusion (or similarly about the functioning of
perpetuum mobile or UFO contacts with aliens)...
Hybrid fusion-fission
nuclear energy
?
Let's imagine briefly (details are not yet known and are now irrelevant), a bit "futurologically", the situation after
the successful management of thermonuclear fusion
and its use in nuclear energy. Fission reactors in nuclear power
plants will be gradually shut down and replaced
fusion thermonuclear. Will the fission reactors be completely
abandoned? It is possible that fission nuclear reactions will
find their "floor" alongside fusion reactions, or
rather the two modalities will coexist or even
cooperate. For chain cleavage reaction even in thermonuclear
fusion D+T play a major role neutrons. And it is
precisely for thoughtful "management" of neutrons that
it could be useful to combine the advantages and
possibilities of both of these methods of obtaining nuclear
energy - to design hybrid fusion + fission
nuclear reactors.
The envelope of a tokamak (or
inertial fusion reactor) would contain uranium-238 or thorium-232
in addition to lithium; by interaction of neutrons it would thus
produce both tritium for a thermonuclear reactor,
as well as plutonium-239 or uranium-233, which would be burned by
chain reaction in satellite fission reactors
with slow neutrons. This would create an interconnected fusion
and fission complex with an optimized neutron balance and a
balanced fuel cycle. From the long-term perspective of
ecologically "clean" nuclear energy without highly
radioactive nuclear waste, however, this solution is probably not
entirely optimal... An exception could be complex hybrid
systems of thermonuclear-controlled transmutation
reactors, ideologically similar to the ADTT accelerator-controlled reactor technology described
above. The "heart" of the system would be a
thermonuclear reactor (such as the tokamak
of Fig. 1.3.8 on the right), which would
not only produce energy, but would also supply neutrons to
control the operation of a subcritically operating fission
reactor. This reactor would contain both fissile materials (235U, 239Pu, 233U) and 238U or 232Th for transmutation
to 239Pu
or 233U
fissile materials. Neutron-controlled fission would
produce energy and at the same time neutron "burned"
("liquidated") hazardous radioactive waste - by converting long-lived radionuclides to stable or
short-lived nuclides (as discussed above in
the "ADTT"
section). However, mastery of these
technologies can be expected only in the distant future (the
problem is, among other things, the neutron deficit)...
Other fusion reactions
The possibilities of using reactions other than
D+T are also being considered, which, however, would require higher
temperatures than we can create so far. These are mainly
reactions of combustion of deuterium itself (D-D fuel cycle) :
D + D ® 3 He + n + 3.3 MeV , D + D ® 3 H + 1 H + 4 MeV .
Deuterium in water is a practically
inexhaustible supply. In addition, fuel cycles by the reaction of
hydrogen and deuterium with lithium, helium-3 or boron-11 could
be considered :
H + 6 Li ® 4He + 3 He + 4 MeV , D
+ 3 He ® 4 He + 1 H + 18.3 MeV ,
H + 11 B ® 3 4He + 8.7 MeV .
Neutron-free fusion reactions - direct
conversion to electrical energy ?
In the fusion reactions discussed so far,
which are suitable for the energy use of thermonuclear fusion, in
particular deuterium-tritium fusion, most of the energy
is carried away by neutrons. We cannot use this
kinetic energy other than through heat - the old
inefficient way of a steam generator, turbine and
alternator. In addition, the massive neutron flux causes unwanted
radiation and induced radioactivity of the
materials. However, there are special fusion reactions (some of which have already been mentioned in several
places in this chapter), in which neutrons
are almost not formed - the so-called neutron-free fusion
reactions. Several such reactions have been
investigated, which have a sufficiently high effective cross
section :
............ table ......
In addition to the deuterium-lithium
reaction (discussed above in ....), special attention was paid to
devoted to proton-boron fusion ..... However,
the kinetic energy of the nuclei of about 600 keV is required for
its efficient course, which corresponds to a temperature of about
6.6 billion degrees. This is not achievable in a tokamak, but inertial
laser fusion pathways are being sought using several
carefully synchronized lasers with very short high-energy pulses
.......
Neutron-free fusion produces energy
in the form of electrically charged particles ( not neutrons as in most fusion reactions) , mostly positively charged helium 4He++ nuclei and photons. This in principle allows direct
electrical conversion of the fusion energy released,
without the need for a thermal-steam cycle, which must be used
for neutrons. These direct conversions of fusion energy to
electric voltage can be based on three basic mechanisms :
- Electrostatic
direct conversion uses the kinetic energy of charged particle
motion (against electric potential) to create a high positive electric voltage at the collecting
electrodes (the opposite effect of
electrostatic particle acceleration). This
electric potential would generate an electric current in the
electrical circuit connected to the electrodes - electrical
power.
- Inductive
based on changes in magnetic fields during the passage of charged
particles. The electromotive force is then electromagnetically
induced in the coils.
- Photoelectric
energy conversion of electromagnetic radiation (in the field of X, optical and infrared), arising during the acceleration and deceleration of
charged particles - braking or cyclotron radiation.
So far, these
mechanisms have only been tested in small laboratory experiments
with artificially accelerated particles and negligible electrical
powers...
Neutron-free fusion
reactions would therefore have the advantage of substantially easier
conversion of energy into electricity and also in lower
induced radioactivity of materials. Some of these
alternative fusion reactions are likely to include the distant
future of nuclear energy ..?..
The "Holy
Grail" of energetics !
Despite all the above problems (and some
others that are likely to arise during development), there is a real hope for the successful implementation
of energy-efficient nuclear fusion in the foreseeable future.
Then the following optimistic statement will apply :
| Thermonuclear fusion: the final solution to the energy problem of humanity |
| Successful management of controlled nuclear fusion opens up a long-term perspective of obtaining large amounts of nuclear energy without accidents and without hazardous radioactive waste |
It would be inherently secure "clean"
energy, without carbon emissions, on a large
scale, that could satisfy the entire planet. And at the same time
save the Earth from a climate catastrophe...
Thermonuclear
reactions in stars
What we have tried hardly and so far little successfully in our
laboratories, has been happening on a colossal scale for billions
of years in nature - in universe. We can see a huge thermonuclear
reactor in the sky every day! And on clear nights, we can see
thousands of such thermonuclear reactors in the sky and with the
naked eye. According to the findings of contemporary
astrophysics, each star, including our Sun, is a
huge thermonuclear reactor held together by its
own gravity - the gravitational pull to shrink the star is
balanced by pressure from heating and radiation during
thermonuclear reactions inside the star (discussed in detail in
§4.1 "Gravity and evolution of stars", part
"Thermonuclear reactions inside
the stars" monograph
"Gravity, black holes and space-time physics"). At high temperature and high pressure,
thermonuclear fusion proceeds very efficiently. Gravity
is also a force that maintains equilibrium operation
of thermonuclear reactions for a long time (several million to
several billion years!): when the reaction
begins to slow and the pressure reduced, gravity slightly
compresses the core of star, pressure and temperature will
increase and reaction starts more quickly; when the reaction
starts too accelerate, pressure increase and against gravity the
star's core somewhat expanse, whereby the pressure and the
temperature is lowered and decreasing the intensity of the
nuclear fusion.
In stars, within
the interior of which there is a huge accumulation of densely
compressed plasma, thermonuclear reactions between hydrogen
nuclei take place sufficiently efficiently *) at temperatures of
about 15 million degrees. However, in our terrestrial
conditions of a small volume of sparse plasma, ten times
higher temperatures are required for the efficient
course of fusion reactions ! - that's why it's so difficult
...
*) For stellar conditions, "efficient
enough" means a fusion energy output of around 300 W/m3. With a huge volume
of hot plasma inside the star, even a small value of power is
enough to cover the energy output of the star and ensure
thermodynamic balance.
The perfect control ability of
gravity for thermonuclear reactions of stars fails only in the
final stages of star evolution, when the basic "fuel"
(hydrogen, helium and other lighter elements) is already consumed
inside the star, the balance is disturbed, oscillations occur and
in the case of material stars, nothing can prevent a
"nuclear suicide" of a star: a catastrophic gravitational
collapse into a neutron star or even a black hole, with
a massive supernova explosion - see the book "Gravity,
Black Holes and Space- Time Physics", §4.2, section "Supernova
Explosion. Neutron Star. Pulsars.". A supernova explosion can
be considered the biggest "nuclear accident"
in the universe!
Possibilities
of obtaining energy from matter
At the end of this chapter on nuclear reactions and the
possibilities of their energy use, we will roughly compare the efficiencies
of individual methods of obtaining energy from matter. We can
compare this efficiency with the ideal situation of the
conversion of all matter m into energy according to the
Einstein relation E = m.c2, which we assign an efficiency of 100%. From this point
of view, the basic methods of obtaining energy from matter will
look something like this (numerical values
are rounded; a more detailed analysis was given in the
picture above - part "Nuclear energy")
:
| actually available ß | ® sci-fi | |||
| chemical reactions (combustion) | fission of heavy nuclei | synthesis of light nuclei | gravity (rotating black hole) | annihilation of electrons and positrons |
| 0.000 000 01% | 0.1% | 1% | max 42% | 100% |
During chemical reactions
(combustion), part of the electrical binding energy
of the envelope electrons in atoms is released; this energy of
valence electrons is relatively small, but is easily achieved by
conventional means in nature. During nuclear reactions
(fission of heavy nuclei and fusion of light nuclei), part of the
binding energy of the strong interaction of nucleons
in the nuclei is released. When gravity is used,
part of the gravitational binding energy of
matter can be released in the field of a very massive compact
object (black holes); however, such objects are not
available in near space. We also do not have antimatter
available.
So far, only the first two items in
the table are practically used, nuclear fusion will hopefully be
used relatively soon (in the order of several decades). There is
no hope for the foreseeable future of the use of relativistic
gravitational energy of matter collapsing into a black hole,
which is a powerful source of energy in quasars (see §4.8 "Astrophysical significance of
black holes" in the book
"Gravity, black holes and space-time physics"). The same probably applies
to the last item - annihilation (see the
relevant discussion in the passage "Antiparticles
- antiatoms - antimatter - antworlds" in §1.5 "Elementary particles").
Nuclear propulsion of space rockets
?
The production of electricity is undoubtedly the main benefit of
obtaining energy from atomic nuclei. Another area in which it is
necessary to supply a large amount of energy in a concentrated
manner is the propulsion of space rockets. Chemical
rocket engines are now used, in which highly exothermic
chemical reactions in the propellant release thermal
energy in the form of hot gases (temperature approx.
2000 °C), which rapidly expand outwards in the
nozzle, transfer momentum and, as a result of the law of action
and reaction, force is created - thrust
propelling the rocket forward. Most chemical rocket engines use liquid
fuel, where hydrogen or hydrocarbons are burned with oxygen,
so the propellant "exhaust" gas is water and carbon
dioxide. The specific impulse - thrust - transfer of momentum -
is given by the speed of the exhaust gas stream. Current rockets
powered by chemical fuel are slow "clumsy snails" (reaching a maximum speed of approx. 20 km/s), not allowing them to reach more distant space objects
in a reasonable time. The use of nuclear energy to propel space
rockets could improve this unfavorable situation.
A
thermal nuclear rocked
The basic, straightforward variant of a nuclear rocket engine is
a nuclear thermal rocket. Here, the chemical
energy for heating the exhaust gases is replaced by the heat
from the nuclear fission reaction. The working gas,
usually hydrogen, is heated to a high temperature
(approx. 2500 °C) in
a nuclear reactor and fed into a nozzle where it expands and
creates thrust. The basic layout is shown in Fig.1.3.11. From the
tank with liquid hydrogen, hydrogen is injected by means of a
turbine pump into the internal space between the fuel cells
of the reactor (where it can also
cooperate as a moderator), where by nuclear
fission is heated to a high temperature, led into the nozzle and
there it rapidly expands.
Fig. 1.3.11. The basic version of the
rocket's nuclear drive - hydrogen heated to a high temperature in
a nuclear fission reactor is fed into the nozzle.
The significantly more effective
ratio between the weight of the consumed fuel and the created
accelerating thrust of the rocket is caused by two circumstances
here :
--> The source of thermal energy is a long-term operating
nuclear reactor, without the consumption of chemical fuel and
oxidizer.
--> The "exhaust" gas of a nuclear thermal engine
is hydrogen gas with a molecular weight of 2, while for chemical
fuels it is water (with a molecular weight of 18) and CO2 (with a molecular
weight of 44). Light molecules have more kinetic energy per unit
mass, so low molecular weight propellant gases are more effective
for thrust than high molecular weight gases. Nuclear thermal
engines with gaseous hydrogen propulsion can achieve 3-4 times
greater specific thrust impulse (momentum
change per unit mass of propellant) than
chemical rockets.
For launching from Earth, when it is
necessary to overcome strong gravity, this nuclear drive is not
usable, due to the higher weight. So far, the start is only
possible with the use of chemical fuel. Nuclear heat engines are
suitable for use only in orbit outside the Earth's gravity and
further in outer space. Outside of the Earth's atmosphere and in
more distant space, there is also no need to address the issue of
radiation protection and radioactive contamination (especially in the case of unmanned flights).
In addition to the usual fissile
material uranium 235U, other radionuclides were also experimentally
proposed. Transuranic americium 242mAm is interesting, which has a very high
effective cross-section of fission by slow neutrons (thousands of barns) and a low
critical mass (approx. 0.1 uranium) --> low reactor weight. It can also maintain a
nuclear fission reaction in the form of very thin metal strips
less than a micrometer thick, from which high-energy fission
products can escape and be used for efficient heating of the
exhaust gas to high temperatures, or in principle also for direct
rocket propulsion. For laboratory experiments, a small amount of 242mAm is obtained in a
nuclear reactor by neutron capture in transuranium 241Am; however, it cannot yet be
produced in sufficient quantities for the chain fission reaction.
In the more distant future,
the use of fusion thermonuclear reaction to
propel space rockets into distant universe is also being
considered. Rather than robust tokamaks, laser-triggered inertial
fusion of deuterium and helium-3 could perhaps be used
here ..?..
All concepts of nuclear propulsion
of space rockets are still only at the stage of ground laboratory
experiments and projects, no such rocket has yet taken
off ...
Energy - life - society - little reflection on energy
consumption
The functioning of all life is conditioned by energy
conversion through complex chemical reactions (see eg §5.2, section "Cells
- basic units of living organisms"). However, man is the only
creature on our planet who, in addition to energy, the source of
which is food, purposefully uses energy from other external
sources. In earlier times, before the era of technical
development, it was the use of thermal energy from a fire, in
which mainly wood was burned, and to a lesser extent the energy
of water and wind (water or windmills). Energy consumption was
low and was drawn from sources constantly renewed by
nature (perhaps with the exception
of ill-considered deforestation); a small
part of the energy of solar radiation, transformed by the
photosynthesis of plants, is enough for this renewal. With the
development of technology (roughly from the 19th century), our
energy consumption gradually increased and had to be drawn mainly
from fossil fuels - coal, oil, natural gas. These fuels,
in which part of the energy from solar radiation, converted and
preserved into a chemical form, has accumulated over a long
period of time, can easily be converted into thermal energy by
combustion and then relatively simply into mechanical and
electrical energy. However, fossil fuels are no longer renewed
(at least not in our time horizon), their supply is not unlimited and there is a risk of depletion.
In addition, a large part of the energy remains unused, due to
the imperfection of the technologies used and the fundamental
limitations imposed by the laws of thermodynamics. Furthermore,
the combustion of these fuels produces a number of undesirable
and harmful by-products *). Finally, the question arises as to
whether the combustion of non-renewable fossil materials is a
sensible conduct, whether it would not be more appropriate to
save these substances and use them as chemical raw materials.
*) From a global point of view,
it is an increase in the content of carbon dioxide in the air and
the related increase in the temperature of the earth's surface
("greenhouse effect"). Many toxic and harmful
substances, acidic combustion products, sulfur and nitrogen
oxides, heavy metal compounds, etc., also escape into the air
when fossil fuels burn, which can have a number of negative
consequences for nature and human health.
It is not generally known that a conventional fossil fuel power
plant also pollutes the environment with the radioactivity
(release of natural radionuclides uranium, radon, and other into
the air), much more than a correctly functioning nuclear power
plant! During coal combusion into the air
released natural radionuclides, especially radon, 222Rn T1/2 = 3.8 days), polonium 210Po (T1/2 = 138 days) and lead 210Pb (T1/2= 22 years), which accumulate
at greater depths underground due to the decay of the primary
natural radionuclides uranium and thorium.
After all, similar phenomena occur during volcanic
activity: the ejected clouds of volcanic smoke and dust
contain a significant amount of radionuclides radon,
poloniun-210, lead-210, from great underground depths. A large
volcanic eruption can be considered, with a bit of exaggeration,
a "natural radiation accident" in
which the extent of radioactive contamination of the environment
is comparable to a nuclear reactor accident (but the isotopic
composition and half-lives of contaminants are completely
different).
At the same
time, the high pace of drawing energy from external sources today
determines all our economic activity and the development of human
civilization. Even our current way of life, highly dependent on
the use of technology. Energy consumption can be expected to increase
further as the Earth's population demands ever higher living
standards. The largest part of the world's energy, more than 80%,
is drawn from fossil fuels, which, in addition to the risk of
depletion, leads to significant pollution of the environment with
harmful emissions. Only a small part (approx. 7%) of energy comes
from naturally renewable sources - hydroelectric and wind, solar
thermal or photovoltaic energy, biomass, etc. Nuclear power from
fission reactors currently covers about 8% of global energy
consumption.
We encounter various, often
conflicting views on the risks and hopes of these individual
types of energy. With regard to the burning of fossil fuels,
almost everyone already agrees that this is not a
promising way of sustainable development. The importance of
naturally renewable resources is sometimes overestimated.
So far, we do not have a sufficiently efficient and affordable
technology to directly convert sunlight into electricity in large
quantities. The mentioned alternative sources, such as hydro and
wind power plants, or solar thermal heating, may be a useful and
welcome solution at the local level, but from a global
perspective they are only auxiliary and peripheral
sources.
The biggest and most tumultuous
differences of opinion appear in nuclear energy.
Here it is necessary to realize that every human activity has its
advantages and disadvantages or risks under certain
circumstances. This also applies to nuclear energy. However, for
an objective assessment, these advantages and risks need to be
assessed in comparison with the advantages and disadvantages of
other, non-nuclear, energy sources. In the lay public, this is
often not reflected, under the pressure of emotional attitudes,
not always sufficiently substantiated by information (or even deliberate distortion of facts by some groups). The benefits and risks of energy from fission nuclear
reactors, where the fuel is uranium *), have been discussed in
detail above in the section "Nuclear
reactors". Some new and perhaps
promising technologies have also been shown there, enabling the
processing of hitherto unusable materials (uranium 238, thorium)
as well as the reprocessing of nuclear waste and reducing its
danger (fast "breeding" reactors, transmutation
technologies such as ADTT).
Realization this technology could also make fission reactors a reasonable
alternative to conventional fuels for many decades.
*) Even heavy natural elements, uranium and
thorium, can in a sense be considered "fossil" fuels:
more than 5 billion years ago, a small amount of energy was
"stored" in the nuclei of these elements by endothermic
reactions during the supernova explosion, from whose products our
Solar System and Earth were formed. We are now drawing this
energy with split the nuclei. The situation is different for
light elements, hydrogen + deuterium (coming from the very
beginning of the universe), where their fusion into helium
releases "new" energy, similar to what stars do.
However, a truly promising and
long-term solution will be the controlled fusion
of atomic nuclei, thermonuclear energy (discussed in detail above in the section "Fusion
of atomic nuclei. Thermonuclear reactions."). The fuel efficiency of
nuclear fusion is about 100 million times higher than that of
chemical reactions (such as combustion). There is enough
fuel on Earth to obtain energy in this most efficient
way available, so this source could provide an almost unlimited
amount of energy even for the distant future. At the same time,
this method is not associated with virtually no risk of
accident, health hazard or significant generation of unwanted
radioactive waste. We are currently throwing hope for the
beginning of its use to the second half of the 21st century.
Rational use of energy
Let's think about the view from the opposite side, saving
energy - "the cheapest energy is saved energy".
The energy savings resulting multiple paths. From a technical
point of view, it is the introduction of modern and efficient
technologies. Examples include microelectronic devices
with low power consumption, which replacing the earlier machines
and apparatus with much higher wattage, or better insulation,
optimization technogical processes, etc.
For reduction in energy
consumption would also significally could contribute, if we revise
the current strong focus on the consumerist
lifestyle and re-evaluated the scale of our life values.
Do we really need to buy a new type of luxury car or large-format
TV every 2 years, change furniture according to the latest
advertisement, build stately villas for many millions, with large
halls and many unused rooms, with pools on large lands? Squander
millions for fleeting "pleasures" and a false sense of
"prestige" and "superiority"? Organizations
and companies, insurance companies and banks behave in a similar
way, with their exhibition buildings with luxuriously equipped
workrooms and offices. We often see a colossal waste of
material and energy, an "devouring,
greed" that nature cannot bear in the long run.
The situation would change, if people came to recognition, that
rather than the consumer "to have", greater joy and
lasting satisfaction brings us the experience of beautiful
unspoiled nature, of discovering new things in nature and the
universe, of a work of art, of friendly coexistence with other
good peoples. Than chassing after consumer "goods", for
which we pay dearly with "our soul"
!
But what we should definitely not
miss energy for are global projects of universal
significance. What would really raise our level not only
materially, but mainly cultural and educational - learning about
the laws and phenomena in nature and universe, improving our
health, harmonious development of society and improving life in
poor areas (but not just importing consumer
goods!). And for what might even save
humanity in the future - eg before the fall of an
asteroid, a deadly flash of cosmic rays and other threats (see §1.6, passage "Biological significance of cosmic
rays", or passage "Astrophysics and cosmology: - human
hopelessness?" in §5.6
of the book "Gravity, black holes and physics of
spacetime").
| Back: Nuclear physics and physics of ionizing radiation | |||
| Nuclear and radiation physics | Radiation detection and spectrometry | Radiation applications | |
| With cintigraphy | Computer evaluation of scintigraphy | Radiation protection | |
| Gravity, black holes and space - time physics Anthropic principle or cosmic God | |||
| AstroNuclPhysics ® Nuclear Physics - Astrophysics - Cosmology - Philosophy | |||