Influence of ionizing radiation on living organisms - risks
and use in medicine
5. Biological
effects of ionizing radiation
Radiation protection
5.1. Effects of radiation on matter. Basic quantities of dosimetry
5.2. Biological effects of ionizing radiation
5.3. Objectives and methods of radiation
protection
5.4. Radiation monitoring and personal dosimetry
5.5. Open radionuclides. External and internal contamination
5.6. Radiation protection in workplaces with
ionizing radiation
5.7. Radiation exposure during radiation
diagnosis and therapy
5.8. Organizational provision of radiation
protection
5.1.
Effects of radiation on matter. Basic quantities of dosimetry
Physico-chemical
effects of ionizing radiation
In §1.6 "Ionizing radiation" we
dealt in detail with the properties of different types of
radiation and its interactions with the substance environment;
however, it was mainly from the point of view of radiation
physics, ie the influence of the material environment on
the propagation of radiation, its absorption, scattering or
conversion to other types of radiation. Here we will deal with
radiation interactions from the point of view of the substance
itself exposed to radiation, ie the effects of radiation
on the physical and chemical properties of the substance. Special
attention will be paid to the effects of radiation on living
tissue.
The very name "ionizing
radiation" (see definition in §1.6) suggests that the basic
physical effect of this radiation on every substance is ionization
- negative electrons are ejected from the originally electrically
neutral atoms, which turns these atoms into positively charged
ions. The resulting effect of this ionization on the irradiated
substance decisively depends on its atomic composition
:
Irradiation of the element - no
chemical change
If the irradiated substance is an element
composed of the same atoms, after the irradiation, the
released electrons are immediately recombined with
positive ions to form again the same atoms of the element
as before irradiation. There are no chemical
changes, may possibly occur minor physical
changes, e.g. the formation of atomic oxygen and ozone
when irradiation of the gaseous oxygen O2, or changes
in the crystaline structure of elements in the solid
phase :
For example,
imagine an experiment if we irradiate a single-crystal of
a diamond. Chemically, a diamond is an element
composed of carbon atoms in a cubic
crystal lattice (this highly regular
crystal lattice causes the diamond's distinct optical
properties). After end of the irradiation, all the
released electrons will "jump" back into the
atoms and it will be pure carbon again. However,
during itself ionization, when electrons are ejected from
carbon atoms, the ionized carbon atoms usually
"jump" out of the bond in the crystal lattice
and do not return there after recombination - they remain
free in the form of amorphous carbon. When
irradiated with large radiation doses, the originally
clear diamond crystal would begin to darken - it
would become a so-called "carbonado - black
mogul" *). At even higher doses (thousands of
Gy), the diamond crystal would eventually disintegrate
into a black powder, "soot". The owner of
a beautiful diamond would certainly not have been happy,
if from this experiment to take a small pile of soot
instead of a crystal ...
*) These dark diamonds called
"carbonado" were formerly found in the West
India, now in Central Africa and Brazil.. However, their
dark coloration is not caused by any radiation, but by
physico-chemical processes during their ancient
crystallization, with an admixture of graphite and
amorphous carbon.

Note: Hihg-energy and very
intense radiation
All this applies to "ordinary" types of
radiation a, b, g of usual energies, where interactions occur at
the level of the atomic shell. However, radiation whose
quantum they have very high energy
(higher than about tens of MeV), and neutron
radiation, causes interaction and changes in the
nuclei of the atoms of the irradiated material -
physical (and induced chemical) changes
occur, including transmutation and
activation of originally non-radioactive
nuclei.
We also do not consider here the situation of extremely high
fluxes of radiation, where the absorbed energy
can lead to melting or evaporation
of the irradiated substance (this can happen, for
example, even with targets irradiated in an accelerator).
Irradiation of a compound ® chemical
changes
However, if the irradiated substance is a compound
formed by molecules of various elements, in particular a
complex organic substance, the ionization of atoms can
lead to a number of chemical changes and
reactions :
1. Ionized atoms are released
from chemical bonds, molecules dissociate.
Decomposition of compounds by ionizing radiation is
called radiolysis.
2. Atoms and molecules released
during radiolysis are usually not neutral, but ionized
and have unpaired electrons - highly reactive
radicals are formed. They can further chemically
react with other molecules of the substance. With their
oxidizing and reducing effects, they can cleave internal
molecular bonds and change the chemical structure of
molecules - new compounds are formed.
The whole "gateway" of new chemical reactions
will open.
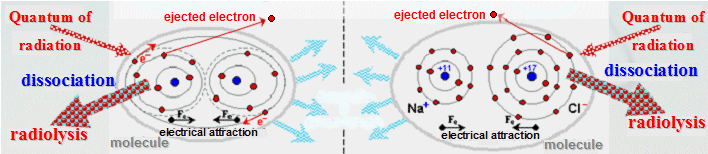
Thus, upon irradiation of the compound, some of the
original molecules are decomposed, whereas new molecules
appear which vere not previously here. The more complex
the irradiated substance, the more diverse the chemical
changes and reactions in it occur. The most complex
chemical substances are contained in living
organisms. Thus, during the irradiation of
living tissue, a wide variety of complex (bio)chemical
reactions occur, which can result in biological
changes at the level of cells, organs and whole
organisms.
Basic
quantities of dosimetry
Dosimetry of ionizing radiation is a field of radiation
physics, which deals with the effects of radiation on substances
in relation to the types and properties of radiation interaction
with matter and the amount of radiation absorbed in matter
(absorbed energy - "dose"). The studied substance is
mainly living tissue, model measurements of
doses and dose rates are performed in water, air and special dosimetric
phantoms. It builds on the physical mechanisms of
interaction of radiation with substances, discussed in detail in
§1.6 "Ionizing radiation".
Absorbed
radiation dose
The degre of physico-chemical effects of
radiation on a substance - as well as the induced biological
effects, if the irradiated substance is living tissue -
it is proportional to the concentration of ions
formed in a given volume of the substance. And this concentration
of ions, in turn, is proportional to the energy
that has been transferred to the substance in
the given volume by radiation.
This default
dosimetric quantity, called transmitted or communicated
energy E, represents the energy [J,
eV] which the ionizing radiation has transmitted to the
substance in a certain volume. It is generally given by
three components: E = SEin - SEout + SEnucl . Here SEin is the sum of the energies of all ionizing particles
that have entered the given volume, SEout is the sum of the energies of all particles that have
left the given volume. SEnucl is the sum of all possible changes in the rest energies
of nuclei and particles that occurred in the considered volume
during the event. nuclear transformations caused by the effects
of radiation (in practice, this third component is usually zero,
with the exception of high-energy radiation of about 10 MeV and
higher). This quantities has only a "school" meaning
and is not used in dosimetric practice...
The basic dosimetric
quantity, which characterizes the physico-chemical and later even
biological effects of radiation on the substance, is the absorbed
radiation dose :
Absorbed
dose (abbreviated
as "dose") D
is the energy of ionizing radiation absorbed at a given
location of the irradiated substance per unit mass. It is
thus given by the ratio
D
= D E / D m ,
where DE is the mean energy of the ionizing radiation
absorbed in the volume element of the substance and Dm is the
mass of this volume element. Unit of absorbed dose is 1
J/ 1 kg, which is called one Gray [Gy]
(sub-unit then 1 mGy = 10-3 Gy and 1 mGy = 10-6 Gy).
This basic
dosimetric unit was named in honor of the English
radiologist R.H.Gray (1905-1965), who in the 30s-50s
intensively dealt with the interactions of radiation with
matter and the effects of radiation on living tissue (including tumor tissue - examined, among other
things, the so-called oxygen effect, mentioned
below in §5.2, the text under Fig.5.2.2). The older unit of radiation dose (in the CGS
system) was 1 rad = 10-2 Gy (rad = radiation
absorbed dose).
Since the majority of the absorbed
energy ultimately changes into heat, the
absorbed dose also characterizes the amount of
transferred thermal energy - heating of
the irradiated material. However, at doses used in
biological applications, this radiation heating is
negligible.
Dose
rate D´
is the dose received at a given site by the irradiated
substance per unit time, ie the ratio of the dose
increment DD for the time interval Dt :
D´
= D D / D t .
The unit is Gray per second [Gy.s-1], in practice the units [Gy/min.] or [mGy/hour]
are used more often.
The size of the dose or dose rate
depends on the intensity of radiation at the irradiated
site (it is directly proportional to the fluention of
radiation), the type and energy of quantum of ionizing
radiation, as well as the properties of interaction and absorption
of this radiation in the substance. Although the radiation dose
is a physical quantity that is basically applicable to
various substances and materials, in radiation dosimetry
it is used mainly in biological applications -
it is mainly determined for soft tissues or water.
And so it is for all the dosimetric quantities listed below.
Note: The
quantity of radiation dose is introduced to express the
degree of usual (obvious, macroscopic, atomic) effects of
radiation on matter - ie absorbed energy, which is manifested by
physico-chemical changes at the level of electron shells, or by
increasing the kinetic energy of the movements of atoms and
molecules in matter. In rare cases, however, the energy of the
radiation can be absorbed by the atomic nucleus in a way that is
not directly reflected at the atomic level. This is especially
the case with neutron radiation, where some neutrons can be
absorbed by the nuclei of the irradiated substance to form a
heavier isotope. This does not affect the normal physicochemical
properties of the substance, but only the increase in the mass of
some atoms. It is debatable whether this part of the absorbed
energy should be included in the radiation dose. The situation is
even more complicated here: nuclei that have
absorbed neutrons are often radioactive (activation
occurs) and during their radioactive transformations considerable
energy is subsequently released, causing a radiation load -
sometimes for a longer time after primary irradiation...
Exposure,
kerma, Terma
When evaluating the effect of indirectly ionizing radiation on
the substance, we can still encouter the quanties of exposure
and kerma, especially in the older literature :
¨ Kerma
(abbreviation of kinetic energy
released in material)
is very similar to the definition of K = DE/Dm and the same unit [Gy] as
the absorbed dose, but for DE takes the sum of initial kinetic energies of the
charged particles released as a result of interaction of the
particles of the primary radiation in the considered volume of
the substance of mass Dm. Kerma is introduced because the basic definition of
dosages comprising only released directly ionizing particles gave
no information on what occurs in the vicinity of the monitored
volume of the substance, especially in the case of secondary
indirect ionizing radiation. For charged primary particles, there
is no difference between the kerma and the dose (for radiation
generated by charged particles, the kerma is not even
introduced). Even for indirectly ionizing radiation in the equilibrium
state *), when the secondary radiation is absorbed, K = D
applies; only in non-equilibrium processes, near the surface of
the substance or at high energies, when part of the radiation can
escape, will K ¹D, while the differences are not large in practice. The
relationship D = K.(1- g) applies between the dose
and the kerma, where g is the fraction of the energy of the released charged
particles that is lost during the radiation processes in the
material. For X-rays and gamma rays with an energy less than
3MeV, the values of both quantities (kerma and dose) practically
coincide, the value of g is only fractions of a percentage.
*) Electron equilibrium
is a state where the energy released by the primary radiation in
a given elementary volume of matter is as great as the energy
transmitted there by the secondary electrons. This balance is
disturbed at the surface of the substance and around the
interface of two different environments. Upon penetration of the
primary indirectly ionizing quantum (photon) into the
environment, the released energy is carried away by secondary
electrons and can be absorbed at a greater depth. At low energies
(X-rays), the outreach of the secondary electrons is very small
and the energy is absorbed almost immediately at the point of
release from the interaction of the primary particle - the
electron equilibrium is met and the dose value is numerically the
same as kerma. In the high-energy g-radiation carries the
secondary electrons to the released energy to a greater depth -
the dose shows an initial rise and only at a greater depth does
the electron equilibrium occur and the dose follows the kerma
with its value.
In the case of
indirectly ionizing radiation (photons g , neutrons), the kerma
characterizes the energy transferred to the charged particles in
matter (electrons and protons), especially during the first
collision. Kerma depends only on the interactions of the primary
radiation in the material of the mass element Dm, while the
absorbed dose also depends on the secondary particles
that were formed around the analyzed volume element and entered
this mass element Dm (in which they have been partially or completely
absorbed). For kerma, it is necessary to specify to which
substance it relates (eg kerma in air or kerma in tissue).
Kerma through interactions
with the medium rather expresses the properties of the radiation
beam, while dose expresses the effect on the
irradiated environment. If kerma is determined in the same given
material environment, mostly in air, it can be used to quantify
the "intensity, abundance" of radiation sources, the kerma
power is proportional to the fluence of the radiation.
In recent literature occurs, although rarely quantity Terma
:
¨ TERMA (Total
Energy Released
per unit MAss of
material) has practically the same definition of T = DE/Dm and the same unit
[Gy] as the absorbed dose. It is a multiple of the mass
attenuation coefficient (m/r) and the primary fluence [MeV/cm2] of the radiation energy at a given location.
If the balance of charged particles is
reached during the interaction of the radiation beam, there is no
difference between the quantities of therma and dose
(or their relationship is linear). However, differences may occur
at the interface of different substances or at the edges of the
bundle.
¨ Exposure
is defined as the ratio of the absolute value DQ of the total electric
charge of one sign ions, which have been released by the
interaction of photons (X or gamma) in a mass element of air
of mass Dm, with complete braking of all electrons and positrons
formed: DQ/Dm, to relate on the unit mass of this air. The unit of
exposure in the SI system is the coulomb per kilogram [C.kg-1] *). Only the charge
of ions released by the interaction of primary photons and the
interaction of secondary electrons released from air atoms is
included in this total electric charge DQ, not including another
charge which may arise from the absorption of braking radiation
emitted by electrons (or characteristic X-rays). For high energy
photons g (higher than 2-3MeV), where the additional ionization
caused by the braking radiation cannot be neglected, the
magnitude of the exposure no longer objectively captures the
effect of such radiation.
*) Rentgen R
The former unit of exposure in the system of CGS
units (Centimeter- Gram- Second)
was rentgen R : It is the amount
of radiation at which 1 electrostatic unit (1 esu »
3.3.10-10 C) of charge is released by ionization in 1cm 3 of dry air (under normal conditions
of temperature and pressure). The conversion relationship is 1R
= 0.258 C.kg-1.
From the exposure value
cannot be determined completly objectively the exact dose of
radiation absorbed by a substance other than air,
because the absorbed radiation dose depends on the properties of
the material and on the type and energy of the radiation. For
gamma and X rays with normal energies of tens to hundreds of keV,
a dose of approximately 0.01 Gray (1 Rad) is
absorbed upon exposure to 1 rentgen in human
tissue. In simple terms, the conversion relationship applies: 1
rentgen » 10 mSv. In the various radiation
tables, the conversion factor between X-rays and Sieverts is
given in the range 1 R = (0.0087-0.0096) Sv.
As with the radiation dose, even at kerma and exposure
are defined the kerm power and exposure power,
as the increment of kerma or exposure per unit time (1 second);
the word "speed" was previously used instead of the
word "power".
Both of these quantities,
exposure and kerma, are mostly abandoned in
dosimetric practice, they are still used marginally in the
primary standardization of radiation beams (eg in radiotherapy,
radiodiagnostics). However, in the literature in the field of
radiodiagnostics and radiotherapy, terminology often persists.
For monitoring X-rays in X-ray diagnostics, eg "input kerm
power in air" is used.
Terminological note:
We use the word "exposure"
in our materials from nuclear and radiation physics in its
general natural-scientific meaning: lighting - the
degree of irradiation of an object or material, exposure
(or exposure time) during
a photograph or other radiation imaging. It has almost nothing to
do with the above-mentioned older abandoned dosimetric quantity!
Radiation
dose from radioactivity
Radionuclides are often used sources of ionizing
radiation in practice (§1.2 "Radioactivity"). Due to the irradiated object, the radioactive
substance can be located either outside - an external
radionuclide emitter, or it can be contained directly inside
the investigated object - internal distribution of
radioactivity.
External
radionuclide source
External radionuclide sources are
most often made in the form of encapsulated closed radioactive
emitters. However, sources of radiation can also be
nearby situated radioactive preparations in vials or
test tubes, as well as a patient with applied activity
for radionuclide diagnosis or therapy. The radioactive emitter
emits its radiation (given by the type of radioactive
transformations and activity) essentially isotropically
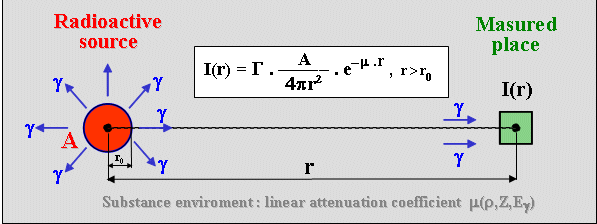
in all directions, up to a full spatial angle of 4p. With the distance
r from the source, the radiation "dilutes", it
is distributed on an imaginary sphere with an area S = 4p r2. Radiation
intensity I (fluence of quanta) emitted by a
radioactive source is therefore directly proportional to the
activity of preparation A and indirectly proportional to
the square of the distance r from the source (this is exactly true for a spot emitter, approximately
in situations where the distance is significantly greater than
the dimensions of the source) :
I
= G . A / 4p r 2 ,
where G is the number of quanta emitted by the radionuclide per
decay.
The same applies to the energy fluence determining the
radiation dose. The amount of energy W[J/s] emitted by a
radioactive emitter per unit time (1s.) is given by the product
of activity A[Bq] and mean energy <E> [eV] of quantum emitted per 1 decay: W = G.A. <E> .1.6.10-19 (coefficient 1,6.10-19 is the conversion factor between energy units [eV] and
[J] ). The radiation dose obtained at time t
, at a distance r from the radioactive source, will
therefore be :
D
= G. (A. <E> .1,6.10-19)
/ (4p r 2 ) . t ,
which is usually expressed in abbreviated form D = G . A/r 2 .t, using the constant G = <E>.1,6.10-19/4p (for a given
radionuclide).
The radiation dose D
from the external radioactive source of radiation is given by a
simple relation *)
D = G .
A/r2 .
t ,
where A is the activity of the source, r is the
distance from the source, t is the exposure time. The
coefficient G is the so-called dose constant
(gamma-constant), indicating the dose rate [Gy.s-1] at a distance of 1
m from a radioactive source with an activity of 1Bq.
*) We mean photon
radiation (gamma) here and spot (or small enough)
emitter, placed in vacuum (or in air), without shielding
materials. It is problematic to use this relationship for
radiation a and b, because part of such radiation is already absorbed
in the source itself and further absorption occurs in air or
another environment lying between the source and the measured
location. We do not consider the half-life of a radionuclide
source here - we mean a situation where the exposure time t
is short compared to the half-life (otherwise
time integration would be necessary, similar to the following
paragraph for distributed radioactivity).
The G- constant
has the base unit [Gy.m2.Bq-1.s-1], but in practice it is most often used [mGy.m2.GBq-1.h-1] - dose rate
[mGy/hour] at a distance of 1m from a radioactive source with an
activity of 1 GBq. The dose G
constant includes the properties of the
radionuclide - the number of photons emitted for decay and their
energy, in relation to the absorption in the irradiated substance
(in water or soft tissue); has different individual values for
each radionuclide. In commonly used units [mGy.m2/GBq.hour], the dose
gamma-constant for some of the most frequently used radionuclides
has the values :
| Radionuclide: |
positron radionuclides
18 F, 15 O, 11 C |
60 Co |
99m Tc |
131 I |
137 Cs |
192 Ir |
226 Ra |
241 Am |
G -constant:
[mGy.m2 .GBq-1 .h-1] |
0.138 |
0.308 |
0.016 |
0.052 |
0.077 |
0.109 |
0.201 |
3.8.10-3 |
In practice, in order to determine
the dose from radionuclide emitters, it is necessary to take into
account the effects of radiation absorption in the
source itself or its packaging, as well as in the environment
between the source and the measured site - the resulting dose
will be lower : I = G. A/4p r2 . e -m
.
r ,
where m is
the linear absorption coefficient of the medium.
Photon radiation
energy ® radiation dose
The energy of incident gamma or X
photons is decisive for the ways of interaction with a substance (§1.6, part "Interaction of gamma radiation and X") and thus also for the
received radiation dose, which is caused mainly by the absorbed
energy of the secondary electrons. Photon
radiation of higher energies has a lower absorption coefficient
(linear attenuation coefficient), but if absorption occurs, the
transmitted energy - dose - is higher. The overall trend is:
higher photon energy leads to a higher radiation dose.
This also applies to the resulting radiobiological effect.
The first ejected electrons may be high energy, but more and more
electron interactions grow into a spray of ultimately low energy
electrons. Thus, harder gamma radiation also produces a larger
number of low-energy electrons, which have a higher LET and
"more time" for the formation of radicals ® higher
radiobiological efficiency.
Internal distribution of
radioactivity
To some extent, the opposite situation to an external
"spot" sealed radioactive source, is a radioactive
substance dispersed (distributed) in the
analyzed or irradiated material, eg in a tissue or organ. This
situation (apart from internal
contamination - see below §5.5, passage "Internal
contamination") occurs especially during diagnostic and therapeutic
applications of radioactively labeled substances - radiopharmaceuticals
- into the body, where these radioactive substances are
then taken up in individual tissues and organs,
according to their pharmacokinetics; is discussed in
detail in §4.8 "Radionuclides
and radiopharmaceuticals for scintigraphy" and §3.6, section "Biologically targeted radioisotope therapy with
open beta and alpha radionuclides").
In this case, the quantum of
ionizing radiation interact immediately with the
substance, instantly after its emission from a radioactive atom.
In principle, all emitted quantum and particles (with the exception of neutrinos)
participate here in the radiation dose - electrons and positrons,
Auger electrons, gamma and X photons, alpha particles, or even
reflected daughter nuclei (§1.2, passage
"Nucleus recoil ").
The charged particles, a or b, have a short
range and give off all their energy near the place of their
emission. In the case of uniform distribution of the radionuclide
in the substance with specific (mass) activity A'[Bq/kg], the
dose rate D' in [Gy/s] from short-range radiation
D'
= A'. <E> .1,6.10-19 ,
where <E> is the mean energy of the emitted particles in
[electronVolts] per decay (coefficient
1,6.10-19
is the conversion factor between energy units [eV] and [J]).
E.g. for the most frequently used
therapeutic radionuclide 131I (for thyroid therapy), the dose from uptake activity
in the lesion weighs g grams is D = 0.109 Gy. g /MBq .h .
Therefore, if
we have homogeneously distributed radioactivity with a time
course A(t) [Bq] in a given area of a substance of mass m, this
will result in a total radiation dose D0-T
[Gy] in this area in time T :
D0-T = 0nTA(t)
dt . <E>. 6.10-19/m
, or D0-T = AS0-T . <E>
.6.10-19/m ,
where AS0-T is the total
so-called cumulative activity (introduced in §5.5, "Internal contamination") in the examined volume at time 0 - T. These relations
are important especially for determining radiation doses in the
body and in tumor lesions during radionuclide therapy (§ 3.6, passage "Planning, monitoring and dosimetry of
radionuclide therapy",
Fig.3.6.11).
Assuming a time decrease of the activity of the
distributed radionuclide according to the usual exponential law
A(t) = A0
.e- (ln2/T1/2ef) .t
with an effective half-life T1/2ef
[s] *), the dose rate will decrease
with the time according to this dependence: D'(t) = A0 .e- (ln2/T1/2ef) .t . <E>.1,6.10-19. The total radiation dose D [Gy],
caused by the radionuclide distributed in the substance, will
then be given by the time integral from 0 to ¥: D = 0ò¥ [A0 .e- (ln2 /T1/2 ef) .t .
<E>.1,6.10-19] dt, which gives the result :
D = A0 . (T1/2ef /
ln2). <E>. 1,6.10-19
.
*) The effective
half-life T1/2ef of
the activity of a radioactive substance is
given by the physical half-life T1/2physical of the relevant radionuclide and in the case
of the organism also by the biological half-life T1/2biol
excretion of the radioactive substance from the given tissue: T1/2ef = T1/2phys .T1/2biol) /
(T1/2phys + T1/2biol). For the kinetics of radionuclides in tissues and
organs, the biological half-life of excretion is usually
dominant, which is usually significantly shorter than the
half-life of the radionuclide used.
If the radioactive substance also
emits high-energy penetrating gamma radiation escaping from the
analyzed volume with distributed radioactivity, as well as in the
case of uneven distribution of the radioactive substance or its
irregular time dynamics, the situation is more complicated and
radiation doses are determined by MIRD (§5.5, part "Internal
contamination").
Determination of the radiation dose from radionuclides
distributed in the organism, tissues and organs is important both
for determining the radiation exposure from
diagnostic applications of radiopharmaceuticals in scintigraphy,
especially in therapeutic applications of open
radionuclides (§3.6, section "Radioisotope therapy").
Radiobiological
efficiency of radiation. Dose equivalent, effective dose
The basic dosimetric quantity - the absorbed dose - does not
include the immediate local distribution of energy
transferred to the substance, which can significantly affect the
specific processes of physical, chemical and especially
biological effects of ionizing radiation. Therefore, another
quantity is introduced which describes the rate of energy loss
along the path of the particle in the substance, and thus also
the rate of braking of the particle and the density of
ions formed along the path :
Linear Energy
Transfer (
LET )
represents the mean energy of a locally transferred to
the substance from flying through a particle, relative to
the unit path of the particle :
L
= D E / D x ,
where DE is the energy delivered to electrons and ions
by a given particle as it passes along the path Dx. The
basic SI unit of linear energy transfer would be 1J/1m
[Jm-1], but in practice a keV/micrometer is used (1
keV.mm-1 = 1,602.10-10 J.m-1). If the
radiation has a short range (alpha radiation), the
absorbed energy is distributed along a short path, the
linear energy transfer is high, so that the ions are very
densely distributed along the path of the particle.
Sometimes the quantity of linear ionization is
also introduced, which is the number of ion pairs related
to the unit path of the particle (eg per micrometer of
the path length).
The higher the concentration of
ions along the path of the particle, the less likely they
are to recombine before they interact with the
surrounding molecules in the irradiated substance - the
more pronounced will be the chemical effect
of irradiation, for living tissue the radiobiological
effect: higher concentrations of ions
and consequently free radicals cause more severe and more
difficult to repair DNA damage (as
will be discussed in detail below).
Since LET describes the local distribution of ionization
at the microscopic level, this quantity is also important
for the analysis of radiation effects at the subcellular
and molecular level - in microdosimetry.
In terms of the
biological effects of ionizing radiation on the irradiated
substance (described below), the radiation is divided according
to the density of ionization, which it induces
in the substance during its passage :
¨ Sparsely
ionizing radiation - X, gamma, beta radiation. For beta
particles with typical energies of hundreds of keV, LET is about
0.2 keV/micrometer. When passed through water or tissue, it forms
about 100 ion pairs /1 micrometer.
¨ Densely
ionizing radiation - alpha radiation, neutron radiation,
proton radiation *). For alpha particles with energies of 4-8
MeV, the LET in the tissue is about 100 keV/micrometer, at the
end of the path in the Bragg maximum it can locally increase up
to 300 keV/mm. It forms up to 2000 ion pairs /1 micrometer of
tissue. Densely ionizing radiation of slower particled has
"more time" to form radicals - higher
radiobiological efficiency.
*) This applies to low-energy radiation, up
to about tens of MeV. However, high energy (about 100MeV) proton
radiation and alpha or heavy ions, however, it is sparsely
ionizing in substances for most of its path. Only at the
end of the path, in the region of the Bragg peak,
the ionization density is high - see Fig.1.6.1 in §1.6 "Ionizing radiation".
As the biological efficiency of different
types of radiation can vary considerably (depending on the
ionization density), for the purposes of radiobiology and
radiation protection, a so-called quality factor Q
is introduced for each type of radiation *), which indicates how
many times a given type of radiation is more biologically
effective than photon radiation - X or gamma (X-ray
with an energy of 200keV are taken as the basis).
*) Also called "radiation
weighting factor" wR or in
radiotherapy "relative biological effectiveness"
RBE (Relative Biological Effectivenes).
The value of this empirical
quality factor Q depends on the type and energy
of radiation. For X, gamma and beta radiation, the quality factor
Q = 1, for neutrons Q » 2-5
(slow neutrons with energy up to 10keV, or again for very fast
neutrons with energy >20MeV), 10-20 (fast
neutrons 100keV-20MeV ), for protons Q » 5
, for alpha radiation is even Q » 20
(as well as for fast heavy nuclei and
fission products).
For a more objective assessment of the
effect of radiation, a "corrected" dose quantity is
introduced with the help of quality factor Q in the field of
radiobiology and radiation protection, which already takes into
account the different biological efficiency of individual types
of radiation :
Dose equivalent
(equivalent dose) in the tissue under consideration is
given by the product of the absorbed dose D at the
given site and the quality factor Q :
H
= Q . D .
The unit of dose equivalent is 1 Sievert
[Sv] *). Dose 1 Sv of any radiation has the same
biological effects as a dose 1 Gy of X-ray or gamma
radiation (for which the quality factor is set at 1).
Dose equivalent is a biophysical dosimetric quantity
that combines the physical quantity radiation dose
with a given type of radiation and an
empirically determined measure of its effect on
living tissue - compared to photon radiation.
*) The unit was named in honor of
the Swedish radiologist R.M.Sievert, who in the years
1920-40 studied the effect of ionizing radiation on
living tissue, laid the foundations of radiation
protection and therapeutic use of radiation. The older
unit of equivalent dose was 1 rem = 10-2 Sv (abbreviation rentgen
equivalent man -
equivalent of biological damage to human tissue). This
unit dates from the 1950s and 1960s, when the
above-mentioned Rentgen unit was used for
radiation exposure. The relationship between
"R" and "REM" is also by means of the
radiation quality factor Q.
As with the
dose, the equivalent dose rate (dose equivalent
rate) is introduced here as the dose equivalent increment
per unit time - the unit is Sievert per second [Sv.s-1].
For radiation monitoring of
individuals, the personal dose equivalent
Hp(d)
is used, which is the dose equivalent at a given location
below the body surface at a depth d
in soft tissue. It is obtained by recalculating the dose
on a dosimeter using the quality factor Q and the
absorption in the tissue according to the radiation
energy.
Different tissues and organs
in the body are differently sensitive to
radiation and their radiation damage leads to differently serious
consequences for the whole organism. Based on statistical
analyzes, the own coefficients of risk of
radiation damage are empirically introduced for each organ and
tissue. Using these coefficients, we can then determine -
estimate - the risk of damage to the body resulting from exposure
to ionizing radiation. For the purposes of radiation protection,
the following quantity is therefore introduced :
- Effective dose of Def - sum of the
weighted mean values of equivalent doses in tissues or
organs of the human body :
Def
= S W T . H T ,
where HT is the equivalent dose in the given tissue T,
wT
is the tissue weighting factor. It is summing over all
tissues over all T considered. The tissue
(organ) weighting factor wT expresses the relative contribution of a given
organ or tissue T to general health damage caused
by uniform irradiation of the body; is normalized so that
the sum of all weighting factors is equal to 1 (S wT = 1).
The values of tissue weighting
factors are given in the relevant tables, eg (according to IRCP recommendation 103) :
| Tissue (organ) |
bone marrow |
intestine |
lung |
stomach |
gonads |
bladder |
liver |
esophagus |
thyroid |
skin |
bones |
brain |
S
-other |
| w T
|
0.12 |
0.12 |
0.12 |
0.12 |
0.077 |
0.04 |
0.04 |
0.04 |
0.04 |
0.01 |
0.01 |
0.01 |
0.12 |
Thus, the effective
dose D ef is calculated using the contributions
of the equivalent organ doses HT of all individual irradiated tissues: during the
summation, each organ equivalent dose HT
is multiplied by its tissue weighting
factor wT,
which expresses the contribution of that particular organ or
tissue damage to whole body damage caused by the effects of
uniform whole body irradiation. With the aid of an effective dose
of Def ,
the effects of irradiating any tissue, organ or part of the body
can be converted into comparable effects resulting from uniform
irradiation of the whole body. The advantage of an effective dose
is that it allows to express the radiation exposure by a
single number (the unit is again Sievert [Sv]) even in
the case of uneven irradiation, or irradiation of only certain
organs, as if it were a radiation load in the case of uniform
irradiation. This makes it possible to compare the
radiation loads of people from various sources - eg from
natural radiation, X-ray examinations, or from different types of
radiopharmaceuticals in nuclear medicine. All of these
assessments relate to the stochastic effects of
radiation - and are only very approximate "qualified
estimates", often of a hypothetical nature,
especially in the low dose range around 0.1Gy (discussed below in the section "Very low dose issues - are harmful or
beneficial ?") ...
Effective dose Def it is
therefore a quantity that assesses the degree of health
risk that arises for a person from the radiation to
which he was exposed. It is an empirical biophysical
quantity that is not directly measurable
- is obtained from the measured radiation dose of a given type of
radiation by taking into account the estimated biological effects
of this radiation and the sensitivity of the individual affected
tissues and organs. It takes into account the fact that different
types of radiation have different biological activities and that
different tissues and organs are differently sensitive, and their
damage has different health consequences for the organism.
However, it should be borne in mind that the concept of an
effective dose is only a very rough and simplified averaged
estimate of the complex and individually dependent processes of
the biological effects of radiation in organisms. It has the
character of a "qualified estimate"
of radiation doses with a number of uncertainties and inaccuracies
of the order of tens of %!
The probability of
biological stochastic effects is proportional to the absorbed
radiation dose [mGy] and irradiated region size [cm3]. In planar X-ray
diagnostics, this is quantified by the quantity DAP
(Dose Area Product) [mGy.cm2], which is the product of the absorbed dose D
and the area S of the irradiated area:
DAP = D. S. In X-ray CT diagnostics, this is quantified by the DLP
(Dose Length Product) [mGy.cm], which is the product of
the absorbed dose D and the length L of the
irradiated area: DLP = D. L. The effective
dose Def [mSv] for a patient, expressing the effects of
radiation on the organism as a whole, is then calculated as the
product of: Def = EDAP . DAP, or Def = EDLP . DLP, where the coefficients EDAP or EDLP include the averaged tissue (organ) weighting
factors wT for structures in the irradiated area (§5.7 "Radiation load in radiation diagnosis and therapy").
To
assess the long-term effects of radiation from internal
contamination with a
radioactive substance - radiotoxicity - the
so-called dose schedule is also introduced, which is the total absorbed dose of
ionizing radiation caused by a given radioactive substance in an
organism, organ or tissue over a period of 50 years from its
uptake into the organism. Radiotoxicity depends not only on the
physical parameters of the radionuclide (half-life, type and
energy of radiation), but also on the chemical characteristics of
the contaminant, which determine the metabolism, distribution to
the various organs, biological
half-life, route of excretion (see
below §5.5, section "Internal
contamination") -
the residence time of a radioactive substance in tissues and
organs.
To assess the
exposure of selected groups of people or populations, a collective
equivalent or effective dose is sometimes used, which is the sum
of the respective doses of all individuals in a given group. These data can be used for demographic
radiohygienic analyzes and to optimize radiation protection.
Physical and
biophysical dosimetric quantities
It is necessary to draw attention to the different nature of
individual dosimetric quantities. Dose, kerma, exposure, linear
energy transfer and some others are physical
dosimetric quantities that
can be (at least in principle) determined on the basis of purely
physical measurements. They describe the objective
measure of the physical
(and possibly induced chemical) effect of radiation on
substances. They can be used not only in biological, but also in
various other technological applications of radiation, where they
determine the degree of required (or undesirable) effects of
radiation on a given material or process. The field of biological
and medical applications is the dominant field of
application of all dosimetric
quantities.
Dose equivalent and effective dose (and
other quantities derived therefrom) are the biophysical
dosimetric quantity
intended for radiobiology and radiation protection
- from basic physical quantities arise by recalculation using empirical
physical-biological factors -
quality factor Q and tissue weighting factors wT. They
are not directly measurable. Approximate values of conversion factors
were determined by radiobiological experiments and based on the results of randomized
clinical trials. It is
necessary to further distinguish which quantities relate to
stochastic and deterministic radiation effects.
Methods
for dosimetric determination of radiation doses
The doses of ionizing radiation are determined by the physical
methods described in detail in Chapter 2 "Detection
and spectrometry of ionizing radiation".
Dosimeters are used, which are simple
radiometers - ionization chambers, GM detectors, scintillation
detectors, film dosimeters, thermoluminescence and OSL dosimeters
- calibrated in dosimetric quantities: radiation
dose [mGy] or [mSv], dose rate [mGy/s] or [mSv/s]. Dosimeters are
used to perform physical measurements (often
using phantoms modeling the tissue environment and
geometric configuration of irradiation),
monitoring workers and patients ("in
vivo" personal dosimetry), radiation
monitoring of workplaces and the environment.
Biodosimetry
Sometimes there are situations where radiation exposure occurs
(or exposure is suspected), without the relevant persons being
dosimetrically monitored - it is irradiation with an unknown
dose. When physical dosimetry is lacking, the
possibility of retrospective dose determination
(or its estimation) arises by monitoring changes in some
biological parameters. Biological dosimetry or biodosimetry
is a radiobiological method that helps determine the size of the
absorbed dose according to the type and intensity of the post-
radiation response of the organism (these
radiobiological effects are discussed in detail in the following
§5.2 "Biological effects of ionizing radiation"). For the reconstruction of doses can be used
some biological parameters, such as changes in blood
counts, occurrence of chromosomal aberrations
or micronuclei in blood cells, observation of somatic mutations
in blood cells, DNA or mRNA damage, increase in p53 protein
concentration, ATM-kinase, .... As adequate treatment needs to be
started as early as 1-2 days after irradiation, it is desirable
to use expression biochemical analysis (statim), such as
ELISA or RT-PCR ...
5.2.
Biological effects of ionizing radiation
As mentioned above, the primary effect of ionizing radiation on
matter is the interaction of the quanta (electromagnetic
or corpuscular) of this radiation with the
electron shell of atoms, occasionally with atomic nuclei. The
result is excitation and ionization of atoms,
which can lead to physical changes and chemical reactions,
and in the case of living tissue to biochemical changes.
These secondary effects can then lead to changes and damage to
the irradiated organism, or even to its extinction - death. From
a biological and medical point of view, the special field of radiobiology
deals with the effects of radiation on living tissue and
organisms. We cannot deal with the medical details of this field
here, they are mostly outside the scope of our physical treatise.
We will mention here only those findings that are important from
a physical point of view, for
applications of radiation in medicine and biology and also from
the point of view of radiation protection methods.
The basic building blocks of all
living tissues are cells. Therefore, in order to
understand the biological effects of ionizing radiation, the
mechanisms of radiation action at the cellular and
subcellular level are crucial. This is related to the
chemical and biochemical effects of ionizing radiation at the molecular
level. Therefore, we will analyze these subcellular
aspects first, followed by an analysis of radiation effects at
the level of tissues, organs
and the whole organism.
Note: However,
these molecular, cellular and tissue aspects cannot always be
strictly separated, so they will sometimes overlap in
interpretation.
Cells
- basic units of living organisms
All living organisms are formed by
cells *) - small formations or "chambers"
bounded by the cell wall (cytoplasmic membrane -
lipoprotein with 2 layers of phospholipids) and filled with
viscous aqueous colloidal solution of various chemicals,
especially complex organic macromolecules of proteins. The field
of biology, studying cells, their structure and function, is
called cytology (Greek cytos
= cavity ).
*) R. Hooke first observed cork cells as early as 1665
using a simple microscope, other cells were more perfectly
observed by A. Leeuwenhoek. Name of the cell
(lat. Cellula ; cella = chamber)
arose from the resemblance to cells in honeycomb. J.E.Purkyne
also made a significant contribution to the origin of the science
of cells - cytology.
Only the discovery of cells and the gradual
recognition of complex biochemical reactions
taking place in cells at the molecular level, at the end of the
19th and in the 20th century, transformed biology and medicine
from descriptive empirical doctrine (description of species,
"counting of petals and stamens", external
manifestations of diseases, ..., with many unsubstantiated and
erroneous opinions) to real science, enabling to
understand the essence and functioning of life
on a uniform exact basis, under physiological and pathological
situations. Research into complex biochemical processes at the
subcellular level will continue for a long time.
Viruses
On the border between living organisms and
inanimate nature, there are viruses (Latin virus
= poison ) - intracellular parasites of microscopic
dimensions of about a hundredth to a tenth of a micrometer. They
consist of nucleic acid (DNA or RNA), encased in a protein shell
( capsid ). Although viruses contain nucleic acids that
carry genetic information in their sequence, they are unable to
grow and produce their own proteins, reproduce, or obtain and
store nutrients and energy - they need a "host" cell to
do so. When a virus enters a cell, it parasitizes on it, uses its
nutrients and can kill it. Or it can copy its genetic information
into the cell's DNA (via reverse transcriptase- see
below) and force ("reprogram") the host cell to work in
favor of the virus. In higher organisms, viral infections are the
cause of many diseases.
However, over the course of evolution, some biological
species have "learned" to use originally parasitic
genetic sequences from viruses for other - beneficial - functions
that have given them evolutionary benefits.
Proteins - the basic building
and functional substances of cells
In the basic filling of the cell, called the cytoplasm (Greek cytos = cavity, plasma = creation ),
more complex structures - organelles -
"float". The liquid part of the cytoplasm, which is an
aqueous gel of large and small molecules, is called the cytosol.
The cytoplasm is composed mainly of a colloidal solution of proteins
(Gr. protos = first , they are biological
substances primordial importance) - they are polypeptide
chains of linear polymers of amino acids.
Amino
acids are molecules containing a carboxyl group
(-COOH) and a nitrogen amine group (-NH2
); these groups and other atoms (hydrogen atom and
side chain) are attached to a central carbon atom.
Amino acids are able to combine with each other by a so-called peptide
bond, in which the carboxyl group of one amino acid
reacts with the amino group of another amino acid. They can thus
form - polymerize - even very long polypeptide
chains - proteins. Of the large number of chemically
existing amino acids, only 24 species (20 basic, 4
special) occurs in cells : 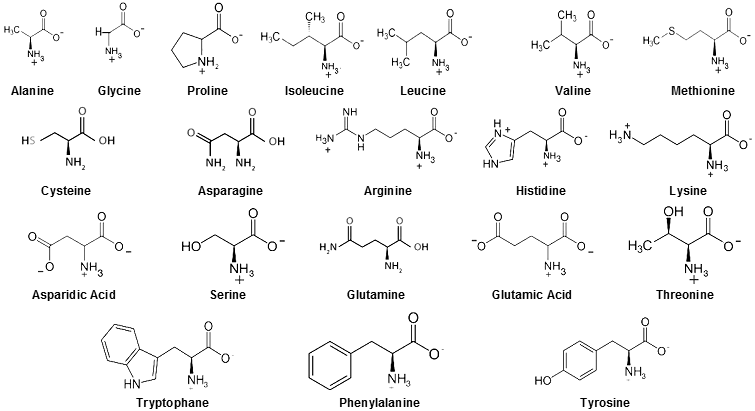
The carboxyl and amine groups, the hydrogen atom and the side
chain are attached asymmetrically (with
the exception of glycine) on the central (alpha) carbon - chiral
asymmetry, which manifests itself in the ability to twist
the plane of polarized light. Depending on the spatial
orientation of the attached groups on the alpha-carbon, the amino
acids can be leftrotatory "L"
or dextrorotatory "D"
(from Latin Leavus = left , Dexter =
right ). Polypeptide bonds can only occur between
amino acids of the same chirality, L or D. In the early
stages of prebiotic evolution, the "left-handed"
chirality of amino acids randomly prevailed, and this preference
then evolved to all biochemical processes. Thus, only leftrotatory
alpha-L-amino acids are present in biochemistry; and natural
carbohydrates are in turn D-dextrorotatory ...
The primary structure of proteins is
given by the sequence of amino acids in the
polypeptide chain ( peptide bond
represents the CN connection between two amino acids, leading to
the presence of the peptide group -CONH-). There
are 20 types of amino acids in proteins, both aliphatic (alanine, glycine, leucine, valine, ...) and aromatic
(with benzene nucleus - phenylalanine, tyrosine,
tryptophan, ...), acidic (aspartic
acid, glutamic acid), basic (lysine,
histidine, arginine), with bound sulfur (cysteine, methionine) and several others.
Individual amino acids have different biochemical properties, so amino
acid sequences in the protein it determines the chemical
properties of the protein and its spatial structure. Linear
polypeptide chains are formed into a spatial (3D) structure due
to the formation of hydrogen bonds between the groups of the
protein backbone - the secondary structure is either regular
repetitive (helical, bending and fiber folding), or irregular and
compact, or more complex joining of different fibers and
sequences (tertiary structure). Some types of proteins are the
basic building blocks of cells, other types of
proteins perform specific functions of cells and
tissues through their complex chemical reactions (as will be
specifically mentioned below in a number of places). The set of
proteins that are contained in the cell or that the cell can
synthesize, defines its ability to perform various biochemical
reactions - processing (decomposition and synthesis) of
chemicals, obtaining chemical energy, creating building
"materials" of cells and other parts of the organism -
it largery detrmines the resulting properties of cells and
organisms.
By
systematically examining of proteins - their origin, structure,
mutual reactions and functions in cells and organisms - deals
with a relatively new scientific discipline called proteomics.
The functional properties of some proteins are discussed below in
the section "Proteins, enzymes,
kinases".
Biochemical reactions - the basis of
cell life
The chemical reactions taking place in cells are controlled and
highly organized, with the basic control role being
played by a complex DNA molecule (deoxyribonucleic
acid, see below), which encodes the composition of proteins
- their primary structure, the sequence of amino acids. Cells
have the ability to metabolize, grow, and make their own
"copies" by dividing one cell into two (for
asymmetric division into stem and effector cells, see below).
The simplest forms of life, single-celled organisms, are
made up of separate cells. Higher multicellular organisms
are formed by a community of a large number of cells, usually differentiated,
where different cells perform their different specific functions,
coordinated by chemical regulatory mechanisms and
"communication systems".
Biochemical reactions usually
involve highly complex organic molecules containing large numbers
of carbon, hydrogen, oxygen, nitrogen, phosphorus and others. The
molecular weight of these macromolecules
in biochemistry and molecular biology is expressed in special
units called kilodaltons (kDa), which is 1000
Daltons (Da). 1 Dalton corresponds to 1/12 of the mass of a
carbon atom, ie roughly the mass of a hydrogen atom, 1Da =
1.66.10-27 kg (J.
Dalton was an English chemist, lived in the years 1766-1844,
significantly contributed to the knowledge of the laws of atoms
and their chemical merging). Thus carbon has 12Da, methane
CH4 has a mass of 16Da, water
has 18Da, glucose C6 H12 O6
has 180Da; the p53 protein has a molecular weight of 53 kDa.
The difficulty of
understanding the function of living organisms
Despite all the advances in biochemistry and molecular biology,
our understanding of how living organisms work at the molecular
level is still very limited. The number, complexity, and
interconnectedness of the biochemical processes that take place
in living cells, still defy our ability to comprehensively
analyze and understand the mechanisms of the
dynamic behavior of these systems.
In addition to the extent and complexity, stochastic
effects are also applied to intracellular systems,
caused by random movements of molecules. Some biochemical
substances are contained in cells only in very small
concentrations, sometimes only units or tens of molecules. With
such a small amount, collisions and reactions of these molecules
occur relatively rarely, and thus with considerable statistical
fluctuations. Such random (stochastic) phenomena can have a
significant effect on the dynamics of biochemical processes,
which can be reflected in the behavior of the whole cell.
Prokaryotic
and eukaryotic cells
Such a complex phenomenon as life has undergone a very long and
complicated development, a number of stages of
which we do not yet know (for the evolution of
life, see the work "Anthropic Principle or Cosmic
God", passage "Origin and evolution of life"). In terms of structure and development,
cells can be divided into two basic groups :
Prokaryote
(Greek: pro = pre; karyon = nucleus
, cells without nucleus, "pre-nuclear"),
whose circular DNA is exposed and floats freely in the cytoplasm
in a structure called a nucleotide. Prokaryotes are the
primitive oldest developmental form of cells, now occurring as bacteria
and cyanobacteria. We recognize two
groups of bacteria: eubacteria, which occur in large
numbers in our environment; archaebacteria, living in an
environment inhospitable to other organisms (hot springs, seabed,
acidic or alkaline environment). The dimensions of the bacteria
range from about 1-10 mm. These
evolutionarily very old cell types form single-celled
organisms, they do not form any functionally differentiated
tissues, they can only associate into colonies.
Eukaryote (Greek eu = normal, correct; karyon = nucleus -
ie "true nuclear") ,
whose DNA (linear, spiral shape) is concentrated mainly in the
cell nucleus, where it is associated
with histones in chromosomes, which are multiple and
contain other chromosomal proteins. Respiratory and metabolic
enzymes are concentrated in organelles, particularly mitochondria
and lysosomes (see below). These cells also have a
so-called cytoskeleton - a system of flexible fibers
(formed by microfilaments and thin hollow tubes - microtubules)
in the protoplasm, providing mechanical support to the cell
structures; they also mediate the intracellular transfer of
complex molecules between the nucleus and other organelles
(during cell division they then form a mitotic spindle).
Eukaryotic cells have relatively larger dimensions, about 5-100 mm. In addition to unicellular organisms,
they form organized communities of multicellular organisms
with cells functionally specialized in tissues and organs,
including humans. In the following text, we will consider mainly
eukaryotic cells, especially somatic cells incorporated
into tissues and organs; prokaryotic cells will be briefly
mentioned in several places for comparison. The figure shows only
a very simplified scheme of cell structure, with enlarged
sections of the cell wall (cytoplasmic membrane), cycloskeleton
(microtubule), mitochondria, nucleus (DNA carried by histone) and
DNA structure (some nucleotides) :

Eukaryotic cell structure. Details of
some structures are schematically drawn in enlarged sections
(frames).
| From a chemical point of view, life
is a very complex system of molecules that can organize
itself , metabolize
using chemical binding energy, grow
, reproduce
and also evolve
over longer periods of time . |
D N A , R N A , proteins
The whole mechanism of cell life organization is
based on a cooperating system of nucleic acids DNA
(deoxyribonucleic acid), RNA (ribonucleic acid)
and proteins: genetic information is stored in
DNA, which is transcribed into RNA, which then serves as matrices
for protein production - below "Proteosynthesis". Different types of proteins are not only the
basic building blocks in cells and tissues, but are also carriers
of cell and tissue function (enzymes).
Nucleic compounds -
etymology of names
:
Latin nucleus = core - here it is a cell
nucleus. Nucleotides are
therefore compounds found in the cell nucleus. Nucleic
acids are then polymeric compounds of nucleotides
showing an acidic chemical nature, in practice caused by bound
phosphoric acid. Ribonucleic acid RNA
is formed by chains of the monosaccharide ribose
bound via phosphoric acid, together with nitrogenous heterocyclic
molecules cytosine, uracil, thymine, adenine, guanine. Ribose
is a 5-carbon pentose sugar; the name comes from Latin ribes
= merusalka, currant, or Arabic ribas = rhubarb - in the
juice of these plants, in addition to acidic components, ribose
sugar is also found. In the cytoplasm of cells there is a large
number of small organelles called ribosomes
(ribo = containing RNA, soma = body - small
bodies containing RNA). In deoxyribonucleic
acid DNA, instead of ribose, its derivative deoxyribose
is bound, in which one hydroxyl group "OH" is replaced
by a hydrogen atom "H".
Note
: This etymology
refers to eukaryotic cells. Similar compounds, although
somewhat simpler, are also found in prokaryotic cells,
where they are scattered in the cytoplasm, there being no
nucleus.
Deoxyribonucleic
acid DNA
A weak acid reaction is caused by phosphoric acid
H3PO4 linked between
nucleotides. It is a very complex long
macromolecule, a polymer of repeating sequences of
deoxyribonucleotides, which has the shape of a double spiral in
eukaryotic cells - Fig. 5.2.2a. DNA contains about 100,000 ¸ 200,000,000 nucleotide pairs,
has a molecular weight of about 2 ¸
100 MDa (megadaltons) (lower limit is for DNA
plastids, mitochondria and prokaryotic cells, upper limit for
human DNA). The "unwound" DNA would have a
linear length of decimetres to meters (in humans
about 2m ..!..); in cells, however, it is very compactly
coiled in chromosomes only a few micrometers in length (see below).
From a chemical
point of view, DNA has a polymeric structure - it consists of a chain
of repeating and interconnected similar building blocks called nucleotides,
which are formed by phosphate H3PO4 (phosphoric acid binding part), deoxyribose
(5-carbon sugar, pentose - monosaccharide 2- deoxy- b- D-ribose; hence the name DNA), purine and
pyrimidine bases (nitrogen heterocyclic
compounds). Nucleotides differ in the representation of 4 various
bases: adenine A , guanine (a purine base) G ,
cytosine C , thymine (pyrimidine base) T . These
four types of nucleotides are arranged in DNA in a certain order
or sequence. This unique grouping of nucleotides
in a strand is the basis of hereditary information
stored in the DNA - the genetic code. They form
triplets, each encoding one amino acid.
DNA thus contains information about the structure of
proteins composed of these amino acids.
The section of a DNA molecule that
encodes the order of nucleotides in a particular functional RNA
and subsequently, through mRNA, also determines the order of
amino acids in a particular protein with a specific function,
"produced" by a cell, is called a gene
(Greek: genos = genus, origin; talent ) *) . Thus, genes provide "instructions"
for protein production - see below "Ribonucleic acid - proteosynthesis".
Although some genes are transcribed into RNA, it does not go
further into proteins; the resulting ribonucleic acids have some
regulatory functions. Overall, however, in eukaryotic cells, only
a small fraction of DNA sequences encode any (functional)
proteins. It is just over 20,000 genes, which represents only
about 5% of the human genome. Most DNA sequences are a kind of
"genetic garbage" - "waste" or
"useless" DNA (junk DNA, noncoding DNA), which
is a relic of the intricate (convoluted) path of evolution
(fossil sequences - "old junk"). They were often
originally "parasitic" genes of viral origin, that were
incorporated into DNA of ancient organisms. Some of them have
been transformed and functionally involved, most have remained
non-functional, others can potentially endanger the organism (perhaps co-operate in the formation of tumors..?..). However, this "unnecessary DNA"
is also in many cases transcribed into RNA, which is a
(protein)non-coding but has a regulatory function
- it determines, for example, the start, stop or intensity of
transcription of coding parts of DNA. On detecting
function of each genetic sequences, including
"unnecessary" (junk) was targeted large-scale project ENCODE (Encyclopedia
of DNA Code Elements; "encode") launched in 2003.
*) Genes,
genetics
Inheritance
- the transmission of some properties of living organisms to
offspring, between generations, has been observed for a long
time. Some biologists (such as J.G.Mendel in the 60s of the 19
centaury, during several years of experiments with the
cultivation of peas with different colors of flowers) empirically
traced some regularities of this heredity, but its true
biological cause remained unknown for a long time. Only the
development of cell molecular biochemistry in
the middle of the 20th century showed, that the essence of
hereditary information is a DNA molecule.
Another structural peculiarity of
genes in eukaryotic DNAs is that the functionally coding
sequences of deoxyribonucleotides - called exons (Greek exo = out ;outer unit)
are interrupted by non-coding sequences, so-called introns
(lat. intro = inside;
inner unit) without genetic content, whose role, probably
regulatory, has not yet been fully clarified. Shortly after
transcription into mRNA are intronic sequences "cut"
(ie. splicing) and thus excluded from the further
process of gene expression; the subsequent joining of the coding
sequences (derived from exons) creates a functional mRNA,
allowing the continuous synthesis of polypeptide chains of
proteins during translation (see below "Ribonucleic
acid - proteosynthesis").
To imagine genetic information only
linearly as a long line of nucleotides - the "letters"
of the genetic code - is simplified and can be misleading;
geometric dimensions and relationships also apply. The double
helix of DNA is intricately twisted and intertwined, so that
parts of DNA that are separated by a long series of nucleotides,
can come into close proximity in the nucleus and then influence
each other's genetic activity.
The nucleotide sequence of DNA in
dividing cells are able to create their own exact copies,
which pass on genetic information to future generations. A
specific form of a gene for a certain trait or property of an
organism is called an allele (Greek:
allos = other), eg a gene for flower color. If a
given organism has the same alleles in its gene pair, it is
called a homozygote, when different alleles then a heterozygote.
The structure of DNA
DNA is made up of two parallel chains (also called strands),
connected by hydrogen bonds between the nuclear bases into a kind
of "ladder" that twists into a double helix. Both
chains are complementary to each other, their
bases are precisely paired with hydrogen bonds. The nucleotide
sequence on one strand is fully determined by the sequence on the
other strand ("positive-negative" pair). These hydrogen
bonds between complementary bases make double-stranded DNA a very
stable macromolecule, despite its complexity.
Only two nucleotide pairs always are associated with each other: C « G
, A « T (Watson-Circk pairing);
these base pairs form a kind of "ladder step" of DNA.
Revealing the
structure of DNA
We cannot directly observe the structure of DNA
molecules, due to the very small transverse dimensions and the
compactification of the llongitudinal dimensions. Under the
microscope, we observe only virtually unstructured chromosomes.
The assembly of the DNA structure was aided by X-ray
diffraction crystallography (it is described in more
detail in §3.3, section "X-ray diffraction
analysis of the crystal lattice structure").
It was possible to grow small DNA single crystals,
that diffractedly reflected X-rays, which created interference
patterns on the photographic medium, which made it
possible to determine the distribution of atoms
in the molecule. A team of researchers R.Franklin, R.Goling,
F.Crick and J.Watson at the MRC Molecular Biology Laboratory in
Cambridge in 1953 completed the analysis of DNA structure and
Crick and Watson created a model that very
successfully explains all cellular genetic processes.
This structure
allows replication: for each of the strings it
is possible to create a new string, completely identical to the
original. During replication, the two strands of DNA move away
from each other, and a new second strand is added to each of
them, nucleotide by nucleotide. Each of the two newly formed DNA
molecules has one strand from the original molecule and the other
newly synthesized. During cell division, DNA is thus able to
accurately reproduce itself and thereby transmit
genetic information to future generations of cells. Replication
is a very precise process in which control and correction
mechanisms are built. Each time a new nucleotide is
added to a new DNA strand, the correct pairing of the previous
nucleotide is checked; if the pairing is not correct, the repair
enzymes immediately remove the faulty nucleotide and replace it
with the correct one. Furthermore, the correct base pairing
between the original DNA strand and the newly synthesized strand
is checked, with the mismatched nucleotide on the new strand
being replaced by the correct one. Thanks to these ongoing fixes,
the resulting replication error rate is only ~10-9 ! Much more often
than replication failures, nucleotides and the genetic sequence
of DNA are damaged by external influences - ionizing radiation
and reactive chemicals (genotoxic
effects discussed in detail below). Cell division and the cell cycle are discussed below in
the section "Effect of radiation on cells", section "Radiation effects during the
cell cycle".
Telomeres
Both end parts of DNA of eukaryotic cells are provided with
so-called telomeres (Greek
telos = end, meros = part ) - marginal
"termination" complexes of repeating sequences, which protect
the ends of chromosomes from unwanted chemical bonds *), as well
as from the "false" evaluation of the end part as a DNA
break (and the initiation of repair mechanisms or apoptosis - see
below). Telomeres have a constant structure of repeating
sequences, very similar in different biological species. In human
and most other eukaryotic cells, it is a short hexanucleotide
sequence [TTAGGG]n of up to n = 2000 repeats.
*) This can be compared to a metal or plastic
reinforcing shoe lace ending that protects them from fraying.
However, in
most replications, there is no complete synthesis
telomere-containing DNA ends - telomere shortening occurs.
The enzyme DNA polymerase, which replicates the DNA sequence, is
not able to copy it completely to the end regions. With each
mitotic division of the cell, the length of the telomeres is
shortened by about 50-150 bases, leading to a gradual reduction
in the protection of the DNA ends; eventually this results in a
loss of mitotic ability of the cells. With excessive truncation,
telomeres no longer adequately protect the ends of chromosomes,
which cellular control mechanisms evaluate and arrest the cell
cycle (such cells remain in the G1 phase of the cell cycle or
undergo apoptosis).
Mitotic shortening of telomeres in DNA is
the main mechanism of the process of "aging" of cells,
called senescence *) - see
also below "Extinction -
death - of cells". Telomere shortening acts as a "mitotic
counter" - eukaryotic cells can only reach a certain limit
in the number of their divisions, the so-called Hayflick
limit (about 50-80 cycles); then they lose the
ability to divide, replicative aging
(senescence) of cells occurs. However, prokaryotic cells that
have circularly ordered DNA without telomeres, can divide
indefinitely.
*) Cell aging is probably a multifactorial
process involving both intracellular "program"
molecular mechanisms and time-accumulating exogenous detrimental
factors affecting cell viability. Progressive damage to cells by
reactive forms of oxygen and nitrogen (radicals) during
metabolism and tissue life is applied, with the ever-decreasing
capacity of antioxidant mechanisms.
Note :
Occasionally there is speculation about evolutionary
significance of
shortening of telomeres, replicative aging and extinction of
organisms: that this process prevents the
overfilling of limited living space by long-lived organisms,
which would slow down evolution. However, this hypothesis is
somewhat debatable for two reasons. 1. First,
replication truncation of telomeres is only one of the mechanisms
of senescence. 2. But most importantly, the
environment has been (and still is) filled with a huge number of
prokaryotic organisms with circularly ordered DNA without
telomeres, where this effect does not apply; and yet the
evolution of eukaryotes has continued successfully... In
interspecies competition and evolution of eukaryotes, however, it
may have some significance..?..
However, there is a
special enzyme called telomerase (it is a complex composed of RNA and proteins:
RNA matrix serves as a template for sequence synthesis of
telomere sequences in DNA, reverse transcriptase transcribes from RNA to
DNA), which is able to synthesize
the terminal sequences of telomeres and thus prevent their
shortening during cell division. For the successful functioning
of telomerase, the enzyme tankyrase is required *),
which causes inhibition of the telomere-bound protein TRF1
("synergistic" action of telomerase and tankyrase).
Such cells are then capable of unrestricted division
(immortilization - "immortality" of cells),
which physiologically takes place only in the early stages of
development of the organism in dividing embryonic cells
and then only in undifferentiated stem cells;
pathologically applied in cancer cells(§3.6 "Radiotherapy",
passage "Carcinogenesis").
*) A complex nucleonprotein system with a
specific function is bound to telomeres. One of the studied
proteins of this kind is TRF1 (Telomeric Repeat-binding
Factor 1), which binds to the ends of the telomere and
prevents telomerase (see below) in its activity to
synthesize the shortening ends of the telomere. However, there is
the enzyme tankyrase, which removes the TRF1 protein and
thus helps telomerase to work...
However, some new experiments show that telomerase is
not the only mechanism for DNA telomere recovery. An alternative
method of "treating" telomeres may be
telomerase-independent inter-telomeric homologous
recombination (see "Reparation
Processes" below), which may lead to elongation of telomere segments,
sometimes several-fold. This method is observed in some plants
and is possibly evolutionarily older than telomerase. Closed
structures, a kind of "telomere loop" at the ends of
the DNA, which can replicate or elongate (mechanisms
of a kind of "rolling circle") have
also been observed. However, somatic effector cells, performing
specialized activity in tissues and organs, lose the ability to
divide indefinitely and no longer divide after reaching the
Hayflick limit.
Note: Mitotic
telomere shortening occurs only in eukaryotic cells with linearly
ordered DNA, where the polymerase is unable to ensure complete
replication of the terminal portions. For prokaryotic cells
(bacteria) with circularly arranged DNA without telomeres, the
limit on the number of divisions does not apply.
Chromosomes
There are two types of DNA in eukaryotic cells: nuclear
and mitochondrial (this is mentioned below in the
section on mitochondria). Nuclear DNA is located in the cell
nucleus in chromosomes (Greek chromos =
color, soma = body) - specific structures about 2-5 mm long, after staining
(with classical Giemsa-Romanowski dyes or fluorescent dyes)
observed in an optical microscope. Chromosomes are composed of
protein carriers, so-called histones (they are small
basic nucleoproteins with a high content of positively charged
amino acids, forming complexes with DNA), around which the DNA
molecule is "wrapped". The chromosomes in cells are
arranged in sets , with the number of chromosomes in one
set (so-called monoploid number x) being normally the
same for each cell in a given organism; in humans it is x = 23
chromosomes. The number of homologous sets of chromosomes in a
cell, called ploidy, is different for different species
of organisms. In humans, most cells are diploid (2 sets
of chromosomes, 23 from the mother and 23 from the father), but germ
cells are haploid (only one set of 23 chromosomes). A
higher number of chromosome sets than 2 is referred to as polyploidy
(triploidy-3, tetraploidy-4, hexaploidy-6, ...); often found in
plants, probably played an important role in evolution. Another
anomaly is the so-called aneuploidy , when a particular
chromosome can be multiplied 3 × or 4 ×, or reduced - 1 ×, or completely lost. Anomalies in the number or
structure of chromosomes, so-called chromosome aberrations,
arise as a result of disorders in mitosis and DNA damage (see
below).
Ribonucleic acid RNA
Another important
"informational" macromolecule in cells is ribonucleic
acid RNA. It is somewhat simpler than DNA, with which it
is similar in many respects: RNA consist of a single
sugar-phosphate polynucleotide strand, which,
however, contains the monosaccharide ribose
instead of deoxyribose. RNA further differs from DNA in that the pyrimidine
base uracyl U occurs instead of thymidine
T. (similar to thymine, uracyl forms a complementary
pair U-A with adenine). The other bases adenine, guanine,
cytosine, are the same in both nucleic acids.
The "World of RNA"
In terms of genetic information in current cells, RNA plays a
"helper" role, although necessary (see below). However,
evolutionary biology concluded that early life was probably based
on RNA (the "RNA world "),
the first simpler molecules of which could have arisen
spontaneously from prebiotic molecules. The original precursors
of the present cells - protocells - were probably just
"clumps" of ribose molecules, polymerized into short
stretches of RNA, coated with water into a simple phospholipid
membrane. Under appropriate conditions, RNA molecules are able to
make copies of themselves and thus "multiply". Peptides
("RNA-peptide world ") may have aided these
processes . Suitable combinations of peptides can produce
enzymes, that catalyze RNA copying. Replicating RNA molecules
encoded properties that were "genetically" passed on to
the next generation. Mutations that occurred by
chance during "copying" led to changes, of which the
"positive" ones allowed these early cells to adapt
better to the environment and thus compete with each other by
natural selection (Darwinian evolution ). It was only
later that a suitable combination of RNA molecules developed DNA,
which, thanks to its greater chemical stability, took on
the role of primary genetic molecule for long-term
storage of genetic information throughout the organism
and for transmission to future generations. RNA began to function
as a "bridge" between DNA and proteins (see below
"Information transfer - proteosynthesis").
Figuratively speaking, RNA passed the "scepter" of
storing genetic information to its duplicate "sister"
DNA and for themselves retained the role of "mediator"
(mRNA). Thus, prokaryotic cells (without nucleus and
without organelles) with cyclically arranged DNA were first
formed; later, more complex eukaryotic cells developed
with a linearly arranged DNA in the form of a double helix
located in the nucleus and with a number of organelles performing
specific functions of cell metabolism.
From the point
of view of evolutionary biology, the questions of the origin
of life, its evolution and desertification, are discussed in
the "Emergence and Evolution of
Life" section of the work
"Anthropic Principle or Cosmic God".
Information
transmission - proteosynthesis
The basic process by which the information contained in DNA is
translated into a specific structure or function is protein
synthesis or proteosynthesis. So-called
gene expression, in which the information stored in the
DNA gene is converted and realized on a specific cellular
structure or function, takes place in two stages. First, transcription
occurs (transcription, copy), when an information or mediator
RNA (mRNA - messenger RNA) is created , the strand
of which is a copy of the base sequence of one of the strands of
the DNA double helix. The transcription of DNA into RNA is
catalyzed by an enzyme called DNA-dependent RNA polymerase.
The single-stranded linear mRNA molecule separates upon
transcription and travels to the cytoplasm. The mRNA is then translated
(transcribed) into a protein, whose amino acid sequence is
encoded by the mRNA as a matrix or template; this second stage -
protein synthesis - takes place in ribosomes (see below "Ribosomes - protein factories"). The information is thus translated from the
"language" of DNA nucleic acids into the
"language" of the amino acids from which the proteins
are assembled. The proteins formed can be used by the cell
itself, or they can travel to other cells, tissues or organs.
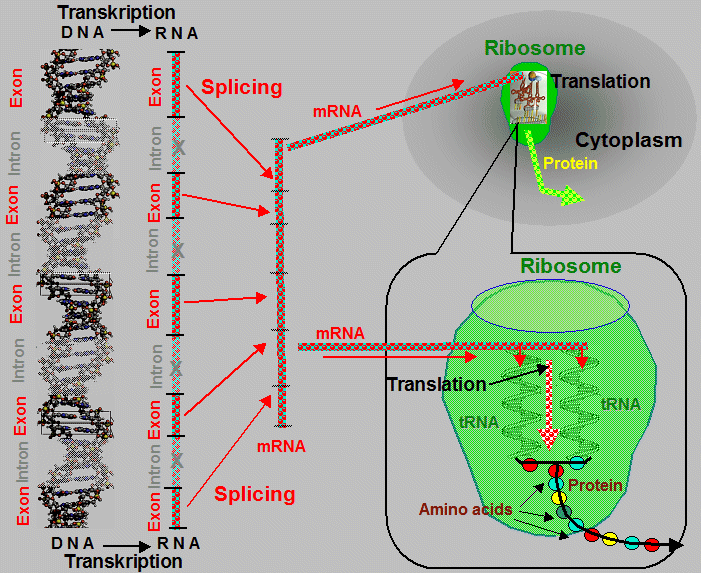 |
Schematic chart of protheosynthesis
in cels. |
RNA splicing
As mentioned above in the DNA section, only a small part of the
DNA sequences encode some (functional) proteins. The coding
regions of the exon represent only small
"islands", which are surrounded by a "sea" of
unnecessary intron sequences.- "genetic jung"
which makes up about 9/10 of the sequences present. Short
sections of DNA that encode proteins are interrupted by long
sequences that do not encode anything and should be removed
before proteosynthesis. The removal of non-coding regions cannot
take place in DNA (whose structure must be maintained as the
genetic information for the next cell generation), but it does so
only at the RNA level by a process called splicing
of RNA. The information contained in the DNA is first transcribed
into a precursor mRNA molecule ( pre-mRNA),
which is an exact copy of a single strand of DNA; it therefore
contains many non-coding sections. During pre-mRNA splicing, the
boundaries between coding and non-coding regions are recognized
by small nuclear RNAs by base pairing. The non-coding portions
are then removed and the protein coding
sequences are recombined into the resulting mRNA
, which enters the cytoplasm (ribosomes) for proteosynthesis.
New research (the ENCODE project) has shown that non-coding RNA
derived from "genetic litter", "unnecessary
DNA", is often used to regulate the expression of coding
genes.
The so-called transfer
RNA participates in the translation process (tRNA), whose function is to transfer amino acids to the
ribosome. The three nucleotides in the mRNA that determine the
inclusion of one amino acid in a protein is called a codon.
In tRNA, it corresponds to a trio of nucleotides that bind to a
codon - the so-called anticodon, which is specific to
each type of tRNA. The transfer tRNA carries in its acceptor part
on the one hand the corresponding amino acids, on the other hand
it has a triple nucleotide (anticodon) which can pair with the
codon of the mRNA. Its molecular mechanism ensures that the
correct amino acids are included in the protein according to the
given sequence of the genetic code. In this two-step proteosynthesis
the resulting proteins then realize the appropriate structure or
function in the cell or organism. It can be said that genetic
information in a cell or organism is expressed in the form of
proteins: the genome (a set of
hereditary information) is transformed into a proteome
(a set of all the proteins in a given organism).
Note: There
is also a reverse process, so-called reverse transcription
, in which an RNA molecule is able to store its gene sequence in
DNA through the enzyme reverse transcriptase. This
occurs when cells are infected by certain viruses ,
whose RNA can thus enter the DNA of eukaryotic cells and alter
their genetic sequence. In a way, the above-mentioned synthesis
of DNA telomeres by telomerase also belongs to this
category of reverse transcription.
After the synthesis
of proteins in ribosomes, their post-translational
modifications can occur - additional chemical
modifications that can give proteins new properties and regulate
their functions. Such a typical modification is phosphorylation
and dephosphorylation (attachment or detachment of the phosphate
group PO4
to a protein), acting as a "switch" between the active
and inactive forms of the protein.
Thus, most genes encode a protein, and
these proteins perform almost all functions in the body.
Furthermore, through proteins, individual genes can interact
indirectly with each other: one gene produces a protein that has (in addition to possibly other functions)
the ability to alter, for example, the
rate of transcription of another gene. This creates a kind of gene
regulatory network with very complex behavior.
Direction of genetic information transfer
Genetic information contained in nucleotide sequences in DNA/RNA
and transformed into amino acid sequences in proteins is
transferred in cells only in precisely defined directions :
1. Transfer - copying - from one DNA to another
DNA. This occurs during replication - cell
division.
2. Transfer from DNA to RNA -
transcription to mRNA - as the first stage of protein
synthesis.
3. Transfer from mRNA to protein -
translation - as the completion of protein synthesis.
4. Reverse transcription from RNA to DNA, in
which insertion (transcription) occurs in the opposite direction.
This is observed when cells are attacked by retroviruses, which
use reverse transcriptase to "sneak" certain sections
of their RNA into the DNA of the attacked cell, thereby forcing
it to create proteins for its own needs. .....
Reverse
transfer from protein to RNA or DNA, as well as transfer from
protein to protein, is not possible. Evolution
has not created any mechanisms for these reverse
transfers. This universal observation for all life here on Earth
is sometimes called the "Central Dogma of Molecular
Biology"..?..
The
impossibility of these reverse transfers of genetic sequences is
the biomolecular reason why the inheritance of
traits and changes acquired during the life of a particular
organism does not apply (this individual inheritance was
assumed by J.B. Lamarck).
Proteins, enzymes,
kinases
Proteins (Greek protos = first; biological substances of
primary importance) - are the basic
material of life. They are the building material
of various structures in cells and tissues and the carrier or
"executor" of many biochemical functions.
These biopolymers consist of amino acids ( 22 basic types of amino acids) linked
by chemical bonds into long chains as "beads on a
string" (as discussed in more detail
above in the introductory section on cells).
For proper function of proteins is important not only the amino
acid sequence - the primary structure, but also the geometric
spatial structure (3D) - the secondary and tertiary
structure into which the amino acid chains are folded or
"packed". The specific (and relatively unchanged,
rigid) three-dimensional structure largely determines the
function of proteins by the "key and lock"
method: in order for a protein to bind to a
specific target molecule or structure, as when a key fits into a
lock. The primary structure, given the amino acid sequence, does
not necessarily determine the geometric arrangement of the
protein. The assembly of proteins into the correct spatial
arrangement is aided by special protein molecules called chaperones
(French chaperon = guard lady) -
they are "guardians" who ensure the correct spatial
structure of proteins, prevent incorrect binding (some chaperones
event. incorrectly packed structures again "unwrap").
Some proteins, however, are not fixedly
arranged spatially shaped, they are unstructured,
flexible - whether as a whole or containing
parts with a fixed structure as well as flexible parts, or they
consist of a three-dimensional shape "as needed". Such
flexible proteins can bind to various types of molecules (they
are "promiscuous"); examples are p21 and p27 proteins.
The known p53 protein also contains unstructured sections.
Proteins that control or catalyze biochemical reactions
or processes are called enzymes (the name comes from the Greek zyme = ferment, yeast
). Each enzyme is "specialized"
for a particular function; enzyme + substrate ® product + enzyme.
The biochemical names of enzymes are usually formed by a base
related to the function and the suffix "-ase":
e.g. lipase (enzyme that breaks down fats into
simpler substances), proteinase (breaks down proteins
into smaller parts, peptides), amylase
(digestive enzyme that breaks down long molecules of starch
(Greek amylon = starch ) into simpler carbohydrates), phosphatase (an
enzyme that releases a phosphate group from its binding
to another compound).
Important enzymes are the so-called kinases
(Greek kineo = move), which are able to activate certain processes and
transfer phosphates to other compounds, acceptors
that have OH groups; a phosphoester of the acceptor
molecule is formed. The transfer and binding of the phosphate
group PO4 (the source of which is most often adenosine
triphosphate ATP, see "mitochondria" below) - phosphorylation
- to a certain site of a protein activates the specific reactivity,
for which the phosphate group supplies energy. Protein
kinases, proteinases, and molecules that regulate their
activity, link the various signaling pathways in
the cell. MAPK (mitogen-activated
protein kinases) is a group of cellular kinases that gradually
phosphorylate with each other to form a network or cascade
leading and amplifying the signal; at the end of the pathway are
substances sensitive to phosphorylation. The pathways usually end
in the nucleus, where they phosphorylate certain transcription
factors.
The biochemical names of some kinases are formed in a
similar way to other enzymes. Tyrosine kinase is
an enzyme that transfers phosphate to the hydroxyl group of the
cyclic amino acid tyrosine bound in the protein. This
affects the function and activity of the protein - it
participates in the regulation of cell growth and division, the
transmission of signals to cells; increased tyrosine kinase
activity (e.g., the epidermal growth factor receptor EGFR) can
lead to tumor growth. For many kinases, the names originated in a
more complex way based on the diseases they were discovered in
reseach. The so-called ATM kinase (Ataxia
Telangiectasia Mutated) is particularly important for our
area of biological effects of radiation, which phosphorylates and
increases the expression of the p53 protein, which acts
as a transcription factor and is essential for cell cycle
regulation and induction of apoptosis (are discussed in more
detail below "Mechanisms of Cell Death", "Apoptosis"). It is also the
anti-apoptotic protein Bcl-2 (B cell lymphoma leukemia-2) and, conversely, the proaptotic Bax (Bcl-2 associated X protein).
Cyclins are
proteins that are present in the cell mainly in a certain part of
the cell cycle; during the cell cycle, there are cyclic changes
in their concentration (hence their name) - expression and degradation, which plays an important
role in cell cycle regulation. Cyclins contain homologous chains
of about 100 amino acids in size.They bind to their partners - cyclin-dependent
kinases CDK. At low cyclin
concentrations, most CDKs are in an inactive free state. As the
concentration increases, cyclins may bind to certain CDK
regulatory sites, causing an increase in their activity - active
CDKs can phosphorylate a number of other enzymes, causing their
activation and deactivation, which can significantly affect cell
cycle dynamics. Due to the complicated regulatory mechanisms in
eukaryotic cells, several species of cyclins and their respective
CDKs are present, regulating different phases of the cell cycle:
cyclins D (regulating the passage of the control node in
the late G1 phase), cyclins E (affecting G1 to S phase
transition), cyclins A (controlling the course of the S
phase), cyclins B (controlling the onset of mitosis and
some processes of its course).
The simpler substances of a
proteinaceous nature are the so-called peptides (Greek pepto = boil, digest; they are formed,
among other things, by digesting of proteins). They also consist of amino acid chains, but in smaller
numbers, about a few dozen (peptides were mentioned at the
beginning of our discussion of cells). They are used for cell
communication, eg in the immune and nervous systems, and include
a number of hormones. This group of substances of
proteinaceous nature includes cytokines (Greek: kytos = cavity, cell; kineo = moving,
transmitting ) produced by cells and
used for their interaction and transmission of information. These
include, for example, interferon (lat.
inter = between; fero = to carry) and
interleukins (the interaction
of white blood cells).
DNA Modification and Damage
Some chemicals can penetrate the cell nucleus, interact with DNA,
and modify it in such a way that it cannot successfully transmit
genetic information during cell division. Such substances can
slow down or stop cell division - they are called cytostatics.
Such affected cells eventually undergo mitotic death,
mainly in the form of apoptosis. Ionizing
radiation (and the chemical radicals induced by it) have
a similar effect, which will be discussed in detail below. Both
of these mechanisms are used to kill tumor cells during
chemotherapy and radiotherapy (§3.6 "Radiotherapy").
A number of chemicals entering cells from
the outside and emerging inside during metabolism or ionizing
radiation, can react with nucleotides and alter DNA sequences -
causing genotoxicity. RNA from viruses
can also enter the DNA of eukaryotic cells (by the action of
so-called reverse transcriptase) and change its genetic
information; so-called oncoviruses may be the trigging
mechanism of mutations, that can lead to tumor transformation of
cells.
Cell wall
protects the cells, separates cells from the
surrounding environment and from other cells, separates the
processes taking place inside the cell, and mediates the regulation
of cellular functions by acting as a selective barrier - membrane.
In eukaryotic cells, the cell wall is formed by a bilayer phospholipid
membrane. Cells receive chemical "signals"
from their surroundings (and possibly from other cells) and
respond to them. For simple bacteria, this is limited to the
perception of increased nutrient concentrations, but for
multicellular organisms work more complex and advanced forms of
communication with the environment, including intercellular
communication. Intercellular communication involves the whole
chain: excretion of a signaling molecule (eg
hormone) from the transmitting cell ® transport
of the signaling molecule to the surface of the target cell ®
registration of the molecule by a receptor on the cell surface ®
transmission of the signal to the inside the target cell ®
cell response - induction of biochemical or biophysical changes
in the target cell. Small hydrophobic molecules similar to amino
acids (such as acetylcholine, dopamine, histamine, ...) can
penetrate the cell directly by diffusion through the
phospholipid bilayer of the plasma membrane - passive
transport. To exchange some ions between the cell and
the environment serves transmembrane proteins,
which form so-called ion channels.
Receptors
On the surface of cells (cell walls) there are so-called receptors
(Latin receptor = receiver of stimulus)
- specific molecules that are able to
recognize other specific molecules in the environment, chemically
bind them and transport them to the inside the cell, or by ion
channel affect the flow of ions through the plasma membrane. A
substance (molecule) that is able to selectively bind to a
cellular receptor is called a ligand (lat. ligo = bind ). The
ligand binds to the receptor and specific signaling to the inside
the cell is triggered. In terms of structure, the transmembrane
receptor consists of three basic parts (domains) :
- The extracellular domain , formed by the
amino-terminal end of the receptor protein, has a ligand binding
site;
- A transmembrane domain that anchors the molecule
in the cell membrane;
- The intracellular domain , formed by the
carboxy-terminal C- termination of the macromolecule, after its
activation triggers signal transmission - it activates other
molecules of the signaling cascade in the cytoplasm, possibly up
to the nucleus.
Receptor activation occurs by kinases
and phosphorylation, as mentioned above in the section
"Proteins, enzymes, kinases". After ligand
binding and phosphorylation of the receptor, the conformation
(spatial arrangement, rotation - other isomerism) of this
molecule changes and the binding site for specific intracellular
signaling molecules is revealed on the inner domain; with the
help of kinases, the activation of other molecules of the
signaling cascade follows. If the chemical signal is transmitted
to the nucleus, transcription factors are phosphorylated, which
in this way induce transcription (or repression) of specific
genes, which then produce the relevant effector proteins
(lat. facio = do, efficio = power).
Cytoskeleton
- skeleton and the tanspoter of cell functions
Cytoskeleton - "cell skeleton"
(cellular "grid" or matrix) - is a system of mechanical
support and "reinforcement" of eukaryotic cells. It
consists of a complex mesh of fibrous protein structures in the
cytoplasm. These structures are of three types :
- Microfilaments (lat. filamentum = thread, fiber) are the thinnest fibrous structures (thickness approx.
5-7 nm), which by their contractility move the cells as a whole,
using the energy obtained by ATP cleavage (see below).
Microfilaments are contained to an increased extend, for example,
in specialized muscle fiber cells.
- Intermediate filaments
are formed by slightly thicker protein fibers (approx. 10-15nm)
and ensure the mechanical stability of cells and tissues against
mechanical stress.
- Microtubules (lat. tubulus = tube, pipe) are the thickest and most complex structures of the
cycloskeleton (diameter about 20-30nm). They are thin hollow tubes
composed of a protein called tubulin, arranged in long
chains of dimeric units in which alpha- and beta-tubulin
molecules alternate. These fibers are helically twisted (13
dimers of alpha and beta tubulin form one complete thread) and
the individual threads are "laterally" bound to the
tube. Tubulin molecules contain binding sites for guanosine
triphosphate (GTP). Tubulin polymerization, associated with
microtubule elongation, occurs by sequential attachment to that
end of the microtubule ("+" end) that contains
beta-tubulin with bound GTP. Hydrolysis of GTP produces GDP (guanosine
diphosphate) and further fiber growth is stopped. The
opposite process to polymerization - dissociation,
consisting in the separation of tubulin dimers from the end of
the fiber containing alpha-tubulin ("-" end), leads to
the gradual depolymerization and shortening of
microtubules. The assembly and degradation of microtubules is
controlled by proteins called MAP (Microtubule Associated
Proteins). Microtubules with their ends ("-") are
"anchored" in the central part of the cell in the
so-called centrosome, bodies in close proximity to the
nuclear membrane, which plays an important role in cell division.
Intracellular movement
and transpost
is one of the essential properties of cells, which is necessary
for carrying out basic biological processes. Targeted
intracellular movement is mediated by the cytoskeleton, to which
a number of special proteins can bind. Along the microtubules, a
number of important molecules are transported in the
cell. It is through special substances called kinesins
that have binding sites for different molecules. Kinesins as
"molecular machines" move - "walk, step" -
along the hollow fibers of microtubules (by means of dinein
"molecular motor" bonds, which contact the microtubules
with rod-shaped protrusions) as along a "cable car" and
thus transfers temporarily bound molecules. To drive each step,
an adenosine triphosphate (ATP) molecule is used, which
alternates with the kinesin terminals, hydrolyzing the adenosine
triphosphate (ATP) to adenosine diphosphate (ADP) and cleaving
the phosphate. The released chemical energy is converted by
electrical polarization into a mechanical force,
causing movements and transport of molecules. In one such step,
the kinesin is shifted by a distance of two tubulin dimers, about
10 nm. In one second, a kinesin molecule can perform about
80 steps.
Unlike the usual concept of a skeleton
(human skeleton, skeleton of a building), the cytoskeleton is not
a fixed and rigid structure, but a highly flexible
and dynamic system that is constantly adjusted according
to the needs of the cell. The changes in the structure and size
of cytoskeleton units are caused by polymerization and
depolymerization (decomposition, dissociation) of proteins into
chains and fibers (with a helical spatial structure - in the case
of microtubules). There is a constant supply of cytoskeletal
proteins (tubulin, actin) in the cell; these monomers then
polymerize into cytoskeletal structures or, conversely,
disintegrate back into monomers. The cytoskeleton transforms
chemical energy into mechanical energy - it is responsible for
all types of cellular movements, intracellular (organelle
movement, cell division) and extracellular (movement of cells,
flagella, cilia ...). Furthermore, the cytoskeleton interconnects
individual cell structures. And not just mechanically. Along the
cytoskeleton fibers, especially microtubules, various proteins
interact and transmit chemical signals, necessary for the
regulation of cellular processes. It mediates the intracellular
transfer of complex molecules between the nucleus and other
organelles, it also participates in cell division (during cell
division it creates mitotic spindle).
In somatic cells, which are part of
tissues and organs, on the cytoskeleton follows up outside the
cell the so-called extracellular matrix: a
system of protein fibrin and collagen fibers (Lat. fibra = fiber , Greek kolla = glue
), connecting the cells and holding them
together in tissues and organs. It ensures the connection between
cells, maintains the integrity and shape of tissues, transmits
movement. The intercellular space itself is filled with a fluid
of gel consistency (interstitial fluid, "tissue
fluid"), which serves to transfer nutrients and oxygen to
the cells and to remove-drainage of metabolic waste products.

Eukaryotic cell structure. Details of some
structures are schematically drawn in enlarged sections (frames).
(this picture is shown here again for
better clarity)
Cellular
organelles
Mitochondria - chemical "power
stations" of the cell
Important organelles in the eukaryotic cell are
mitochondria (Greek mitos =
fiber; chondros = grain) - are
important organelles in a eukaryotic cell. They have a diverse
shape from elongated fibers to compact grains, about 0.2-5
micrometers in size. The number of mitochondria depends on the
metabolic activity of the cell: in average cells
there are several hundred of them, cells with higher energy
consumption requirements can contain several thousand
mitochondria, in metabolically exposed cardiomyocytes there are
up to 100,000 of them and they occupy more than 30% of the cell
volume.
They have two membranes, inner and outer :
- The inner membrane is irregular, many
times bent (depressions into the interior
are sometimes called krists). It
is selectively permeable only to molecules involved in the
respiratory chain and oxidative phosphorylation. Multiprotein
complexes are located on the inner membrane, which mediate
cellular respiration by proton and electron transport; cytochrome
c is particularly important for our interpretation (see "Apoptosis" below).
- The outer membrane resembles
a cell wall and is permeable to most small molecules, but not to
more complex protein molecules (such as cytochrome c). It
developed during the phylogenetic development of the plasma
membrane of aerobic bacteria after their endocytosis (see below).
The main function of mitochondria is the
production of chemical energy in the form of molecules
ATP (adenosine triphosphate) C10H8N4O2NH2(OH)2(PO3H)3H. ATP molecules, which are "energy
transporters" in cells, are formed in mitochondria by
oxidation of citrates and fatty acids (Krebs cycle, hydrogen
oxidation, oxidative phosphorylation). At the site of need, ATP
is then oxidized to ADP (adenosine diphosphate + P) or AMP
(adenosine monophosphate + 2P) to release considerable energy
(approximately 57 J/g). Mitochondria are sometimes referred to as
"power stations" of cells in which the oxidation of
nutrients occurs, especially glucose, to form carbon dioxide,
water and output ATP, whose energy is used in cells for
endothermic processes - macromolecule synthesis, transport of
molecules against the gradient, cell movement (including muscle
contraction), formation of electrical potentials in membranes,
heat production. Mitochondria are formed (multiply) in cells by
continuous binary division ("budding", similar
to bacteria) independent of the cell cycle; inside the inner
membrane they contain their own mitochondrial DNA,
completely different from nuclear DNA.
Evolutionary
origin of mitochondria
Mitochondria have an interesting evolutionary origin.
Ancient eukaryotic cells, when developed from prokaryotes, had
no mitochondria. They ate, among other things, the
various prokaryotic cells they fed on. About 1.5-2 billion years
ago, the original eukaryotic cells sometimes phagocytosed
some species of ancient aerobic bacteria (similar to
protobacteria of the order Rickettsiales), which,
however, did not decompose and digest, but left them intact -
they established endosymbiosis with them *). The host cell obtained oxygen and nutrients from the
environment, which they passed on to these bacteria - future
mitochondria - which metabolized them into energy molecules for
their own needs as well as the needs of the whole cell. Such a
symbiosis has proven to be mutually beneficial.
Over the millions of years, the host cell has completely
"domesticated" its bacteria, the endosymbiot has given
up most of its independence and become a mere organelle. Circular
DNA, similar to prokaryotic nucleotides, is still present in
mitochondria and ensures their partial genetic autonomy. In plant
eukaryotic cells, chloroplasts (a kind of photochemical
"battery") occur instead of mitochondria, whose similar
evolutionary origin is derived from endosymbiosis with cyanobacteria.
*) Trace phylogenetic
"evolutionary tree" of eukaryotes to
the "root" is very difficult. In addition to the primary
endosymbiosis somewhere in the beginning, several other
secondary or tertiary endosymbiosis occurred during evolution.
Comparative analysis of morphological characters
are often misleading. A more reliable method is now molecular
phylogenetics, which has developed procedures for
reconstructing phylogeny according to the similarity of genes and
proteins. By comparing gene sequences (most often genes for RNA
small ribosome unit, SSU rRNA, which are present in all cells) in
different organisms, certain similarities can be traced, which
may be indications for their phylogenetic relatedness. The
problem is that during evolution, gene fusions of originally
separate genes very often took place and, conversely, gene splits
into two new parts. Therefore, it is necessary to perform
phylogenetic studies on multiple genes and proteins - multigene
or phylogenomic analyzes using sequencing of genomes and
mediator mRNA molecules, followed by statistical analysis of
similarities.
Lysosomes
Other organelles in the cell are lysosomes
("decomposition bodies" - "digestive
vesicles"). Digestion is the enzymatic breakdown of
ingested nutrients into basic biological building blocks, which
are amino acids, monosaccharides and fatty acids. In lysosomes
the chemical decomposition - hydrolysis - of various
more complex organic substances (sugars, fats, nucleic acids,
proteins) takes place in an acidic environment with many
different enzymes. The products of this decomposition can then be
processed in mitochondria to form ATP energy molecules,
that "distribute" the binding chemical energy needed to
synthesize complex substances and for other cell functions. And
in ribosomes for the creation of specific cellular proteins.
Lysosomes are about 0.1-1 micrometer in diameter, and there are
several hundred of them in cells; a larger number are found in
the gastrointestinal tract - liver and pancreatic cells.
Ribosomes -
"factories" for
proteins
Ribosomes (ribonucleon-protein particles) are small
organelles found in large numbers in the cytoplasm of cells and
also on the surface of the endoplastic reticulum. They are about
20-30 nanometers in size. The number of ribosomes in cells is
highly variable, proportional to the growth rate of the cell. In
most cells, there are on the order of 10,000 - 100,000 ribosomes,
but in some cases in eukaryotic mammalian cells there can be up
to 10 million. Ribosomes have only a temporary existence in
cells, a relatively short one. After the production of a specific
protein is completed, the two subunits separate and usually
decompose. In eukaryotic cells, new ribosomes are formed in the nucleolus,
where RNA synthesis and ribosome biogenesis take place. In
prokaryotic cells, this process takes place in the cytoplasm,
where RNA molecules travel.
Ribosomes are composed of RNA
and proteins, structured into two subunits. The small subunit of
the ribosome has a molecular weight of about 800 kDa and consists
of 20 proteins and an RNA molecule of about 1600 nucleotides in
length (16S RNA). The large ribosome subunit is about 1500
kiloDaltons in size and consists of 33 proteins and two RNA
molecules (23S RNA of 2900 nucleotides in length and 5S RNA of
120 nucleotides in length). In ribosomes, proteins are
synthesized from the mRNA strand by translation
- decoding information from the mRNA according to which the
resulting amino acid sequence is assembled; tRNA molecules are
used for "reading", which carry an amino acid on the
one hand and a triple nucleotide (anticodon) on the other hand,
which can be paired with an mRNA codon (as described above).
Specific nucleotides of 16S RNA from the small subunit of the
ribosome form hydrogen bonds with codon and anticodon nucleotides
only when properly paired and geometrically oriented. The actual
peptide covalent bond, which connects two amino acids, is formed
in a large subunit of the ribosome, where the so-called
peptidyl-transferase center containing 23S RNA and ribosomal
proteins is located. Ribosomes are a kind of cellular
"construction lines" or "factory" for protein
production: nuclear DNA ("design office")
supplies informational mRNA ("technical drawing"),
which in ribosomes translates into the amino acid sequence of
proteins (using a transfer tRNA for each amino acid) -
runs its own "production" of a specific protein
molecule. It was discussed in more detail above "Information
transfer - proteosynthesis".
Other organelles
A relatively large formation in the cell's is the endoplasmic
reticulum (in the microscope it
appears as a kind of "clot" in the cytoplasm) in the shape of a coiled membrane sheet. It consists of
two types: a rough (granular) reticulum whose surface is
dotted with ribosomes (these granules cause
"roughness") and a smooth endoplasmic
reticulum with a network of channels connected to the rough
reticulum. These units play a central role in the synthesis of
proteins, lipids, steroids and other substances in the cell. Golgi
complex is endomembrane system vesicles in the vicinity
of the endoplasmic reticulum, used to transport and modification
of proteins.
Cell
specialization
Multicellular organisms consist of cells of different species
that have different shapes and different activities - they are
"specialized" to create various
differentiated tissues and organs with a specific function, or to
mechanically build, for example, a supporting or locomotor
system. These specialized cells, performing a certain function,
are called effector cells (Latin
effector = executor). There are
about 230 species of various specialized cells
in the human body. These are, on the one hand, body cells - somatic
(Greek sóma = body ) - these are practically all cells in the body, and
sexual cells - gametic (Greek
gameté = woman, wife)) - in
mammals they are eggs and sperm.
To ensure growth and
regeneration, stem cells (maternal, clonogenic)
are also present in the tissues and organs. Stem cells are
capable of unrestricted division, being able to produce both
identical stem and daughter cells, bearing the characteristics of
a given differentiated tissue - asymmetric division
into two different cells, an identical stem and a different
daughter cell *). Asymmetric division ensures the process of differentiation,
in which the emerging cells by their structure and function adapt
to the specific role they are to play in the organism. All cells
in the organism are provided with the same genetic equipment, but
as a result of this process, only a certain part of genetic
information is realized in a particular cell (due to specific transcription
factors, only certain genes are transcribed in the
cell, others are not applied). Daughter effector cells usually
enter the quiescent G0 phase of the cell cycle.
*) Cell division and cell
cycle are discussed below in the section "Effects of radiation on cells", section "Radiation effects
during the cell cycle".
New research has shown that specialized
effector cells can, under certain circumstances (eg due to stress
from an unfavorable environment, such as a change in the acidity
of the environment), lose their specialization
and become cells that have all the genetic equipment available.
The division of these cells may appear to be involved in the
regeneration of damaged tissues; it is also speculated that some
cells, after losing specialization due to mutations, may
transform into clonogenic tumor cells.
Extinction
- death - of cells
Extinction of
living organisms
No organisms do not live forever - they are "lethal".
The causes of extinction (death) of organisms
can be very diverse, but we can roughly divide them into two
categories :
1.
External causes
- mechanical destruction, attack and "eating" by
another organism, thermal damage, chemical or radiation damage,
adverse change in external conditions, lack of nutrients.
2. Internal
factors - physiological disorders and diseases,
"wear and tear" of organs and degeneration of vital
functions, aging - senescence.
External and internal factors are often closely related.
Infections, radiation or chemical pollutants, even if the
organism successfully copes with them in the acute phase, can
initiate latent processes of internal degradation, which can
eventually cause the extinction of the organism ...
Aging of organisms
Aging - senescence - of a living
organism is a complex irreversible process by gradually reducing
the efficiency of vital functions and decaying the body's
structures. It is an integral part of the life of all
multicellular organisms and will eventually result in their
extinction. The rate of senescence is a fundamental limiting
factor for the life span of organisms. This
maximum life span is very different for each
species of organism.
The longest living
organisms are some conifers - Sequoia sempervirans or Pinus
aristata, reaching 3000-5000 years. Examples of animals are
long-lived turtles such as Chelonoidis nigra
(approximately 200 years) or bivalves Arctica islandica
(500 years). Most animals have a life span of years or decades,
in insects they are usually months, the shortest is in mayflies
(max. 3 days).
Aging is a multifactorial
process involving various mechanisms, of which three are probably
the most important :
- >
Reactive free radicals, especially oxygen, formed during
many metabolic processes in the body. The main source of free
radicals in cells are mitochondria, in which
cellular respiration and the production of chemical energy take
place. Although antioxidant enzymes are present in
organisms, the ability to produce them decreases with age. Free
radicals then begin to accumulate more and can damage cells and
tissues (especially causing DNA strand
damage, as described in the following section).
- >
Accumulation of DNA defects arising spontaneously and
accidentally during life due to replication errors, oxygen
radical reactions, or mutagenic reactions (which
usually enter cells from the environment with food). Some of these more serious defects in important
genetically coding parts of DNA are not repaired, they accumulate
in the organism, disorders of proper function of cells in tissues
can occur. It is observed that the number of spontaneous
chromosomal aberrations in somatic cells increases with age.
- >
Telomere shortening during mitotic cell division (described above in the section "DNA, RNA, proteins, chromosomes, telomeres", passage "Telomeres"). After exceeding a certain critical length, the
telomeres lose their protective function of the terminal parts of
the DNA and the cell can no longer divide; usually programmed
cell death - apoptosis - occurs. The regular shortening of
telomeres during each division means that somatic cells can
perform only a limited number of divisions during their lifetime
- about 50-80. This number of divisions is
called the Hayflick limit (Hayflick
and Moorhead found out in 1951) .
Therefore, the cells capable of dividing are becoming less and
less in the organism over time, the functional capacity of the
tissues decreases and therefore the organism ages - replicative
aging.
Note: The
aging process - senescence - is sometimes related to the
2nd law of thermodynamics - growth entropy
(disorder) of closed systems. However, this 2nd law of
thermodynamics is only a hidden physical
framework lying deep in the molecular background. However, the
real explicit mechanisms here are the biochemical processes of
free radicals, DNA defects and telomere shortening during cell
division.
Mechanisms of cell
death
At the cellular level, we encounter four basic
types - mechanisms - extinction and inactivation:
apoptosis, autophagy, necrosis and senescence of cells.
"Mitotic catastrophe" is sometimes mentioned as a
special category. Under physiological circumstances, eukaryotic
cell death is one of the mechanisms for maintaining tissue
homeostasis balance in the production of new cells and
the extinction of old, redundant, damaged cells. It can also
serve as protection against mutated cells, which can be dangerous
for the body.
Cell
apoptosis
The basic way of controlled (programmed) death of damaged,
dysfunctional or otherwise redundant cells in multicellular
organisms is called apoptosis (Greek apoptosis = falling - the name comes
from the fall of leaves caused by the death of cells in the
petioles) - a kind of cellular
"suicide". It is a complex chain of processes
influenced by a number of factors and controlled by many complex
biochemical molecules, especially proteins and enzymes. Apoptosis
can be induced by two types of stimuli :
¨ Internal (intracellular)
activation of apoptosis - irreparable DNA damage,
toxic substances in the cell (eg oxidation products), lack of
nutrients. Apoptosis triggered by internal mechanisms acts
as a protection against the survival and proliferation of
defective cells, especially mutated cells with damaged DNA.
¨ External (extracellular) activation of
apoptosis - binding of certain substances (ligands
of "death"), transmitted by regulatory mechanisms of
tissues and the organism, on the relevant receptors of the
cytoplasmic membrane. Properly functioning apoptosis,
triggered by external regulatory mechanisms from the tissue,
helps maintain tissue homeostasis and provides effective
protection against excessive cell proliferation.

Above: Internal and external signaling
pathways of apoptosis activation. Bottom:
Morphological changes of the cell during apoptosis.
Internal signaling pathway
of apoptosis
An important role in apoptosis, especially in the process of
internal activation of apoptosis, plays the p53
protein (having a molecular weight of 53
kilodaltons and a length of 393 amino acids).
Under normal circumstances, its level in the cell is low
(maintained by the mdm2 protein ), which is sufficient
to maintain cell cycle regulation. DNA damage activates ATM
kinase, which triggers the production of DNA repair
proteins and (via mdm2) activates and phosphorylates the p53
protein, which acts as a transcription factor. In the early
stages, p53 can activate DNA repair (via GADD45 proteins
) and slow down the cell cycle (via p21 proteinen coded
by the WAF/CIP1 gene) so that repair can occur over time. The p21
protein inhibits cyclin-dependent CDK kinases and
arrests the cell cycle in the G1 phase; in the case of proper
function of p53, the cell cycle is restored only after the
elimination of errors in DNA. If p53 detects DNA damage beyond
repair options, its expression is increased, thereby inducing the
synthesis of Bax and other
mitochondrial-binding proteins; at the same time, the
anti-apoptotic Bcl2 protein (mentioned below) is
inhibited. Mitochondrial membranes lose potential and become permeable;
cytochrome c *) is released from mitochondria, DIABLO
protein (direct IAP binding
protein with low pI) , AIP (Apoptosis-Inducing Protein) and the caspase chain is started (see below).
*) Cytochrome c , if it
is inside the mitochondria - located on the inner membrane
(through which it mediates electron transport), is important for
cellular respiration (there is cytochrome-c-reductase and
cytochrome-c-oxidase). However, if released from the
mitochondria, it becomes a proteolytic cell poison that can lead
to cell death.
Thus, the p53 protein (discovered in 1979 in the British Cancer Research) has the function of a cell cycle regulator and a kind
of "guardian of the genome" :
- It can activate DNA repair, while temporarily
pausing the cell cycle to allow time for repair mechanisms;
- If it turns out that the cell's DNA is so severely
damaged that its repair is no longer possible, it can trigger
apoptosis, which programmatically leads the cell to
"safe" extinction.
In this way, the organism is protected
from potentially dangerous cells with a damaged genome, ie also
against tumor cells. Hence the name tumor-suppressor gene TP53
for the corresponding DNA sequence (located at 17p13.1), which
encodes as a product (transcription factor) the p53 protein.
Mutations in the TP53 gene can lead to tumor growth (along with other factors, see §3.6 " Radiotherapy
", section "Carcenogenesis"). Several other genes (cooperating and competing) are
also involved in the function of TP53.
External signaling
pathway of apoptosis
External activation of apoptosis is initiated by the arrival of a
"deadly" ligand that binds to the
appropriate "deadly" receptor located
on the cell surface. One such ligand is the so-called TRAIL gene (the name comes from the field of oncology: tumor
necrosis factor-related apoptosis-inducing ligand ). Below the cell surface, pro-caspases are then
activated (8 and 2), the process branches to the inhibition of
the mitochondrial membrane (and the release of cytochrome c
as in the above-mentioned internal activation) and to the direct
activation of effector caspases (-3,6,7).
Caspase chain
The actual phase of irreversible apoptosis is caused by the
activation of proteins from a group of cysteine proteases
called caspases *) (abbreviation caspase =
cysteinyl aspartate-specific protease), which
break down intracellular proteins (proteolytic degradation),
including the cytoskeleton and cell matrix; this then leads to
the morphological changes of the cell mentioned below. They also
attack the nucleus and cleave DNA.
*) So far, 14 species of
caspases are known, of which about half are involved in
apoptosis. Some of them, so-called pro-caspases
(-2,8,9,10) in inactive form, commonly occur in the cytoplasm. By
reacting these "signaling" caspases with cytochrome c,
or association with receptors, effector caspases
(-3,6,7) are expressed, which break down proteins and other
macromolecules in the cell.
After starting the apoptotic
process the DNA cleaves into smaller sections,
hydrolysis of cellular proteins, degradation of cycloskeletal
structures and organelle membranes takes place. Furthermore,
there is a membrane depolarization of the cell
wall, the exposing of phospholipids on the cell surface,
increased permeability of the plasma membrane, and
later the violation of the integrity of the cell wall. In the final phase, the cell shrinks and breaks down
into several smaller fragments (apoptotic bodies), on the surface
of which molecules get from the inner wall (eg
phosphatidylserine - PS) stimulate their phagocytosis
- "ingestion" by surrounding cells, phagocytes or
immune cells (macrophages -
increased exposure of PS on the cell surface is an attractive
signal for macrophages: "eat me! "). Thus, in contrast to necrosis, there is also a
regulated and gentle "clearance" of cell death
products.
Apoptosis is the main mechanism of
biological action - cell death - when irradiated with low
and medium doses, tenths to units of Gy. In the
induction of apoptosis by ionizing radiation (internal signaling
pathway), the mechanism of its occurrence and time dependence
differ significantly in different cell types. Rapidly dividing
radiosensitive cells can enter apoptosis from different points in
the cell cycle, less radiosensitive cells show varying lengths of
"blockage" at some stage of the cell cycle, mostly in
the G2 phase. At this time, the cells do not divide and are given
time to repair the damage. If the damage is irreversible,
apoptosis is initiated. The total time that cells have available
to repair DNA damage before eventual entry into apoptosis is an
important factor in the radiosensitivity of these cells at lower
doses (2-5Gy). At higher doses (approximately 20 Gy), ionizing
radiation-induced apoptosis occurs independently of cell blockage
at a certain stage of the cycle, and the time that
apoptosis occurs after irradiation depends on the radiation dose
received.
An interesting effect accompanying the
apoptosis of specific irreversibly damaged cells is the so-called
bystander effect (discussed below), leading to apoptosis
or genetic changes in some surrounding cells, which were
not originally damaged.
Inhibition of
apoptosis
Antiapoptotic mechanisms may also be involved in cells
and tissues. Some mutations in the TP53 gene (deletion or point
mutation) can cause inhibition of apoptosis. Apoptosis can also
be blocked at the mitochondrial level. The antiapoptotic gene Bcl-2
(B cell lymphoma / leukemia protein 2 - was isolated
from tumor cells of lymphoma B), which is found in mitochondria
and whose increased concentration protects mitochondrial
membranes - prevents the penetration of cytochrome c and
the triggering of the caspase chain of proteolytic degradation in
the cytoplasm (blocks the effect of p53 protein). Bcl-2 is
intensively expressed by its BCL-2 gene during embryonic
development; in adulthood it occurs only in stem cells (and in
tumor cells).
Both Bcl-2 and BAX are transcriptional targets for an
important regulatory protein p53. Expression of the pro-apoptotic
Bax protein can be induced by ionizing radiation,
cytotoxic agents (such as chemotherapeutics), or other genotoxic
stress.
Cell
autophagy
This is a kind of "self-ingestion" of a cell (from the Greek auto = himself ; fago = eat, devour
). During complete autophagy
(macroautophagy) of cells, autophagocytosis, the
walls of lysosomes are disrupted and the cell content is
subsequently degraded (decomposed, "digested").
Autophagic vacuoles form in the cell, latter the process of
autophagy can affect the whole cell. Autophagy can be induced by
cytotoxic substances or radiation, under physiological conditions
it acts as an adaptation mechanism to a lack of nutrients or
oxygen. Partial autophagy- a controlled process of
self-cleavage and digestion - occurs when a cell tries to survive
adverse conditions by digesting part of the cell cytoplasm or its
less important organelles. Autophagic vesicles are transported to
lysosomes, in whose acidic environment complex biological
molecules decompose, recycling energy and necessary substances.
This maintains protein homeostasis during adverse conditions;
after their subsided, the cell can continue to function and
divide.
Cell
necrosis
The direct death (extinction) of
cells, which is practically not controlled by cellular
mechanisms, is called necrosis (Greek nekros = dead
). It arises as a result of severe
irreversible damage to cells - mechanical damage, chemical
damage, strong overheating or hypothermia, hypoxia, strong
radiation. Oxidative phosphorylation is stopped, ions accumulate
in the cell, cell edema (enlargement) occurs, organelle membranes
rupture and proteolytic enzymes are released into the cytoplasm.
Protein and DNA are broken down, the cell wall is broken, and the
degraded cell contents spill into the extracellular space, which
is accompanied by an inflammatory reaction in the tissue.
Necrosis can affect entire groups of adjacent cells (tissue
necrosis).
When irradiated with ionizing radiation, cells
necrosis occurs only at very high doses of tens
to hundreds of Gy.
Cell
senescence
Cell aging ,
called senescence , is a long-term multifactorial
process involving both intracellular "program"
molecular mechanisms and time-accumulating exogenous detrimental
factors affecting cell viability. Progressive damage to cells by
reactive oxygen and nitrogen during the life of tissues is
applied, with the ever-decreasing capacity of antioxidant
mechanisms. Cells can only reach a certain limit in the number of
their divisions, the so-called Hayflick limit (about
40-60 cycles); then the cells lose their ability to divide. The
limited replication potential of cells is mainly due to the
shortening of the so-called telomere - they are
marginal complexes in DNA that protect the ends of chromosomes
from adverse reactions and binding and from their evaluation by
cellular mechanisms such as DNA breaks. During DNA replication,
there is no complete synthesis of the DNA ends, so at each cell
division, the marginal ends - telomeres - are shortened in the
daughter DNA. With excessive truncation, telomeres no longer
adequately protect the ends of chromosomes, which evaluate cell
control mechanisms and stop the cell cycle. Telomere shortening
thus functions as a "mitotic counter". After reaching
the Hayflick limit, cells age, replication senescence
occurs (cf. also the passage "Extinction - death - cells" above) .
However, there is a special enzyme called telomerase
(it is a complex composed of RNA and
proteins, containing sequences serving as a template for the
synthesis of DNA telomere sequences - reverse transcriptase), which is able to re-synthesize the terminal sequences
of telomeres and thus prevent their shortening during cell
division. Such cells are then capable of unrestricted
division (immortilization -
"immortality" of cells), which physiologically takes
place only in dividing embryonic and stem
cells, while pathologically it is applied in tumor cells
(§3.6 "Radiotherapy", passage "Carcinogenesis").
Senescence can be accelerated by some
harmful chemical factors, as well as exposure to ionizing
radiation (aging of skin cells accelerates excessive sun
exposure) - premature stress-induced senescence (a
regulatory pathway of senescence, independent of telomere
shortening leads through the p16 protein).
Entosis
of cells
Entosis of cells (from the Greek. entos
= inside
) is a phenomenon where two cells connected
to each other and will merge in one cell,
temporarily or permanently - one cell is "internalized"
inside another, a "cell within a cell" is formed. In
laboratory cell colonies, it is occasionally observed under a
microscope that two cells gradually approach each other,
"entering" each other, occur "drawing" one
living cell into the cytoplasm of another cell. In many cases, it
is "cannibalism", where one cell absorbs another, which
disappears due to lysosomal enzymes - this could be classified as
cell death (as is the case with phagocytes).
However, sometimes endosymbiosis occurs, when
the absorbed cell survives, it may even divide inside the
"host" cell, or it may leave it again without damage.
Entosis occurs in cells that have separated from
binding in the extracellular matrix. As such a released cell
approaches close to another cell, adhesion forces to the
cytoskeleton of a neighboring cell begin to act on it, which can
push it inward. So far, the question is whether entosis is a
phenomenon that actually occurs in the body (where cells are
fixed in the tissues in the extracellular matrix) or only in free
cell cultures..?.. Entosis could play a role in cancer. It is not
yet clear whether positive (another type of cell death that can
help reduce tumor cells) or negative (tumor cells can
"hide" in other cells from immune mechanisms or
chemotherapeutics and then relax again and begin to
multiply)..?..
Mitotic
catastrophe
This is sometimes referred to as a more complex combined
mechanism that may (but may not) result in cell death. It occurs
as a consequence of erroneous mitosis when a
cell attempts to divide without proper repair of damaged DNA (as
in Figure 5.2.2c), due to failure in the G2/M control node and
premature activation of CDK1. In a mitotic catastrophe, a larger
number of multipolar dividing spindles and decondensed
chromosomes appear, nuclear fragmentation or loss of the nuclear
membrane occurs, two or more nuclei may be present (multinucleation)
caused by an inaccurate distribution during cytokinesis. This
situation can lead to "secondary" mitotic cell death,
mainly by the mechanism of apoptosis or autophagy. However, if
the process of apoptosis is inhibited, asymmetric multipolar
division may occur, resulting in aneuploid cells with gene
mutations or chromosomal instability.
Note: Faulty
mitosis can also occur due to the action of certain chemicals
that attach to the microtubules and prevent them
from polymerizing or depolymerizing. Depolymerization of
cytoplasmic microtubules and polymerization of new microtubules
(astral, polar, kinetochore) around centrosomes at the nuclear
membrane, forming a dividing spindle, is one of the key
articles in cell mitosis. The respective anti-tubulin substances
(microtubule inhibitors) are therefore referred to as mitotic
poisons. Of the natural plant substances, it is, for
example, colchicine (an alkaloid contained in ocun),
which causes inhibition of microtubule polymerization, or taxol
(the alkaloid paclitaxel contained in yew), which causes
inhibition of depolymerization. However, the targeted
intervention of microtubular inhibitors in cell division can be
used to stop the growth of tumor cells (§3.6 "Radiotherapy",
passage "Chemotherapy").
Immune
death of cells
In multicellular prokaryotic organisms, complex interactions of
various specialized cells occur. During evolution, the immune
system evolved, whose task is to protect the body from foreign
cells - especially infection by external intruders, but
also from its own damaged or mutated cells. ... The main
biochemical molecules mediating immune protection are proteins
from the group of immunoglobulins . .
Although immunoglobulins can also induce apoptosis, the main
mechanism of immune killing of unwanted or
"suspicious" cells is the activation of
complements - membrane glycoproteins C1-C9, which by
their proteolytic effects attack cytoplasmic cell membranes and
they cause their penetration. The cell
dies and released chemicals cause an inflammatory
reaction with leukocyte accumulation. Unlike apoptosis, this is
the external proteolytic destruction of the
cell. Another mechanism of immune cytocides is the induction
of phagocytosis - the fixed Fc region of bound antibody
specifically binds to the Fc receptor of some types of
leukocytes, especially macrophages, which then recognize and
subsequently phagocytose tumor cells. ...
These cell killing mechanisms are used in biologically
targeted therapy of cancer using monoclonal
antibodies (§3.6 "Radiotherapy",
passage "Monoclonal antibodies") .
Further details of the structure of cells and their
chemical and biological functions lie beyond the scope of this
physically focused treatise. We will therefore turn to our main
topic - the biological effects of ionizing radiation :
Mechanisms
of the effect of radiation on living matter
Free radicals
One of the basic chemical phenomena in the irradiation of
substances with ionizing radiation, especially substances
containing water and more complex compounds, is the formation
of free radicals. Free radicals are those atoms and
molecules, that have one or more unpaired electrons in the last
orbit of the electron shell. Such an atom or molecule is then
highly unstable and reactive *). It tries to
reach an equilibrium state by obtaining another electron
"into a pair" from the surrounding molecules. In this
reaction, a molecule that has lost an electron can become a new
radical. Free reactive radicals, due to their oxidative and
reducing effects, are able to cleave various
types of internal molecular bonds in biomolecules and degrade
thus their chemical structure, necessary for the respective
biological functions.
*) In addition to free radicals,
some other substances whose molecules do not have unpaired
electrons show similar properties of high reactivity. It is, for
example, hydrogen peroxide H2O2,
hypochlorous acid or atomic oxygen O1. These substances are
also formed during irradiation and contribute to biological
effects (especially in the increased presence of oxygen - see
"oxygen effect" below).
Free radicals enter into many reactions in
the biological environment. It is, for example, lipoperoxidation
of fats (with the formation of aldehydes), oxidation of
proteins, glycation proteins with glucose, changes
in RNA and DNA strands, that can lead to cell death or
to mutations (see below).
Over millions of years of
evolution, organisms have partially "learned" to use
free radicals even to their advantage. Examples are white blood
cells containing precursors and enzymes that are capable of
generating free radicals; they then participate in the
elimination of bacteria in the phagocytes. In terms of the
biological effects of radiation, however, we will deal mainly
with such reactions of free radicals, that lead to cell
damage.
Certain "opponents"
of free radicals are antioxidants. These
substances either prevent the formation of free radicals, or the
free radical preferentially oxidizes this substance, which
counteracts the oxidation inside the cell. The best known
antioxidants are ascorbic acid (vitamin C), uric
acid (prevents the formation of hydroxyl radicals in the
blood plasma), cysteine, folic acid ; elements such as selenium,
magnesium, zinc, chromium, ...
Effect of irradiation
on cells
If we want to outline the effects of
ionizing radiation on cells, it is necessary to consider two
basic situations separately :
× Strong irradiation (with a dose of
hundreds of Gy) => decomposition of biochemical molecules, denaturation of
proteins in the cytoplasm and organelles, cessation of all vital
functions, immediate cell death (in interphase) - cell
necrosis.
× Weaker irradiation (tenths to units of
Gy) => negligible effect on cytoplasm and organelles,
radiobiological effect on DNA can result in mitotic
death of cell - apoptosis, or change in
genetic information - mutations. Or, thanks
to repair mechanisms, there will be no effect.
In our treatise, we will deal with
radiobiological processes especially in common situations of
small and medium radiation doses of tenths, units or tens of Gy.
We begin with the effects of individual radiation quanta.
 "Cell.gif" image
"Cell.gif" image
So what happens when an incoming quantum of ionizing radiation enters
into the cell ? According to the "Cell.gif"
image (which we have shown here again for clarity), a eukaryotic cell has a complex structure, so it
depends on which specific part of the cell is affected by the
quantum of radiation :
--> If the quantum of radiation hits the cytoplasm
and damages some protein in the cytosol, this molecule is soon
removed in the lysosome and nothing happens.
There are a large number of protein molecules in the cytoplasm
and they are constantly supplemented by proteosynthesis in
ribosomes.
--> When the quantum of radiation hits a some
cellular organelle - lysosome, mitochondria,
microtubulus, this structure is soon replaced by a newly formed
one and essentially nothing happens again.
--> However, if the quantum of radiation hits the
cell nucleus and the deoxyribonucleic acid DNA
in it, a number of serious biological effects
can occur, discussed in detail below :
Dominant
effect of radiation on DNA, genotoxicity
As mentioned above, ionizing radiation causes chemical and
biochemical changes in living tissue, which can generally damage
all parts of cells, cytoplasm, individual organelles. However,
the response of cells to radiation is determined primarily by the
behavior of DNA. Nuclear deoxyribonucleic acid
(DNA) is the most biochemically important macromolecule in a cell
- it contains basic information about the structure and function
of a cell. Intervention in the biochemical structure of DNA can
cause a cell to stop producing the necessary protein, or it can
produce altered "foreign" proteins that do not fulfill
their function (sometimes they can even be
toxic).
DNA macromolecules are the dominant
"targets" for the biological
effects of ionizing radiation - there may be mainly breaks in the
sugar-phosphate chain and base changes. E.g. oxidation on an
amino group such as adenine produces an OH group to which
cytosine binds instead of thymine - an error
occurs in sorting the amino acid into an string.
On the DNA double helix, the radiation can cause a number of
damages (see Fig.5.2.2b), the main ones being two types of
interruptions or "breaks" :
× Single strand
break (SSB) ,
damaging only one strand (string) of DNA. These breaks are
usually easily repaired by the cell using the enzyme DNA
ligase.
× Double strand
break (DSB) ,
affecting both strands (strings) of DNA. Here, repair is much
more difficult and often unsuccessful. A double break in the
structure of DNA often leads to the death of a cell - to its lethal
damage, it usually disappears by apoptosis (it
was described in detail above in the section "Mechanisms
of cell death").
Mechanisms for repairing DNA damage will be discussed
below - section "Repair
processes".
The DNA fragments, micronuclei
When the double stranded DNA breaks occur, these breaks often
fail to repair. A large DNA fragment may then
separate from the original DNA double helix, which remains in the
cytoplasm and during cell division may enter the cytoplasm of one
of the daughter cells. Micronuclei are fragments
of chromosomes, encased in a nuclear membrane. They tend to be
about 1/10 the size of a cell nucleus and are well visible under
a microscope after chromatin staining.
During radiation exposure, other
biochemical molecules in the cytoplasm, cytoskeleton or
organelles - lipids, carbohydrates, proteins and others - are
also damaged in the cells. However, a large number of these
molecules are contained in the cells (and they are continuously
supplemented by the synthesis of other molecules) and a more
pronounced effect of radiation would occur only when a larger
percentage of these molecules were damaged; this occurs only at
relatively high doses of tens and hundreds of Gy. However,
significant DNA damage occurs even at lower doses (tenths to
units of Gy), when the effect on other biochemical molecules is
still small. Therefore, in explaining the mechanisms of the
effects of ionizing radiation on cells, we focus primarily on the
effects on DNA.
Note: Some
new radiobiological studies using very thin
"micro-bundles" of charged particles (mainly alpha and protons, but it was also proven on
X-rays) have shown, that in some cases the
direct DNA damage is not necessary to trigger intracellular
damage mechanisms. Even in the case of cytoplasm
irradiation, a kind of "remotely induced"
response (bystander response) can sometimes occur, leading to radiation damage
to cells - apoptosis or genetic changes (cf. the "Bystander effect"
discussed below).
Since DNA damage leads to disruption
or change of genetic information in the cell, we talk
about the genotoxic effects *) of ionizing
radiation and the chemicals induced by it in cells. Overall,
ionizing radiation acts as a cytostatic (cell
damage, cell division arrest) and mutagen
(damage or alteration of genetic information). For the harmful
effects of ionizing radiation on the organism, individual tissues
and organs, the collective name radiotoxicity is
used.
*) Genotoxicity
is also shown by a number of chemicals, that
enter cells from the outside (through the body's metabolic
pathway) or are formed inside the cells (during internal
metabolism of cells or at ionizing radiation). These substances
enter the cell nucleus, react with DNA and cause deterministic
(mitotic cell death - cytostatics, stronger irradiation) or stochastic
effects.(mutations, tumor formation - long-term effects of
carcinogens or weaker radiation); see "Dose-biological
effect relationship" below. Also some viruses
(retroviruses, oncoviruses) have a high genotoxicity:
the RNA of these viruses is able, by the action of reverse
transcriptase, to enter the DNA of eukaryotic cells and
change their gene sequence.
No specific genetic changes or mutations are known,
that can be attributed only to the effects of
ionizing radiation and distinguish them from changes caused by
chemical action (external or internal). The genetic effects of
radiation are manifested only by an increased frequency
of spontaneously occurring mutations and genetic changes.
Basic
stages of the effect of ionizing radiation on the organism
First, we in a overview analyze the radiation effects from the
global point of view of the whole organism (in
the next explanation, we will return again to the inductive
procedure: intracellular mechanisms ® cells ®
tissues ® organs ® organism). The process of the
effect of ionizing radiation on living tissue takes place in four
significant stages, differing in their speed and mainly in the
type of ongoing processes; the relevant processes are
schematically shown in Fig.5.2.1 :
- Physical stage
During the interaction of a quantum of ionizing radiation
with matter, the energy of radiation is transferred
mainly to orbital electrons in atoms with the formation
of ionization and excitation (the relevant processes are described in §1.6). If the released electrons have a high enough
energy, they can create a whole cascade by exciting and
ionizing other atoms. At an absorbed dose of 1Gy in the
tissue, approximately 105 ionizations are generated in the volume of each
irradiated cell (typically about 10 mm in size). This
primary process is very fast (practically instantaneous, the speed of quanta
is equal to or close to the speed of light), it only takes about 10-16
-10-14 seconds.
- Physico-chemical
stage
Ionization and excitation lead to the disruption
of chemical bonds between atoms and molecules.
Secondary physico-chemical processes of ion
interaction with molecules occur, during which molecules
dissociate and free radicals
are formed (eg from water H2O forms hydrogen cations H+ and hydroxyl anions OH- and
unstable products capable of oxidation of H2O2, HO2). Even this
process is very fast, it does not take longer than 10-14 -10-10 sec.
- Chemical stage
The formed ions, radicals, excited atoms and other
products react with biologically
important organic molecules (they "attack" DNA,
RNA, enzymes, protein molecules) and change their
composition and function. A typical disorder at the
molecular level is chain breaks in the DNA
molecule - either a break in just one strand of
the sugar-phosphate chain, or a complete break in the
double-stranded DNA. Furthermore, damage to purine and
pyrimidine bases, atypical binding "bridges" (cross-lines)
inside the double-stranded DNA, local denaturation and
other chemical changes can occur - Fig.5.2.2b. The
individual "genotoxic" processes of
this chemical stage last for various lengths of time -
from thousandths of seconds to of the order of units of a
seconds, depending on the transport time of the reactive
components from the place of their origin to the place of
localization of the attacked biomolecule.
- Biological stage
Molecular changes in biologically important substances
(DNA, enzymes, proteins) can result in functional
and morphological changes in cells, organs and
in the organism as a whole. The length of time for this
phase ranges from a few seconds at the cellular level, a
few days in tissues and organs, to years at the level of
the whole organism. The diversity and longer timing of
biological changes are related to the complexity of
biochemical and metabolic processes in living organisms
and to the action of a number of feedback mechanisms. At
the level of the whole organism, the biological stage can
appear somatically after a few tens of minutes at high
doses of radiation, at medium doses within a few days -
acute damage or radiation sickness due to the destruction
of a large number of cells. However, at low doses, it may
involve a latency period of several years or even decades
(late stochastic effects). Specific types of biological
effects of ionizing radiation are described in more
detail below.

Fig.5.2.1. Schematic diagram of significant processes and their
time sequence in the effects of ionizing radiation on living
tissue.
Note: The
scale on the time axis is basically logarithmic, but in some
sections it is slightly modified so that it is possible to
clearly draw individual events.
Only the physical and chemical stage depends on
the physical parameters of the radiation, while
the subsequent radiobiological response of the cells is
determined only by the biological properties of
the specific cell types. It can be seen from Fig.5.2.1, among
other things, that in most cases the interaction of quantum
ionizing radiation with living tissue has no effect.
It is when :
J Occurs recombination of free radicals
before they just react with biologically important substances;
J Repair mechanisms at cell level successfully repair
DNA or other substance damage;
J Cells killed by radiation are quickly replaced
by the division of healthy cells - compensatory cell
proliferation;
J The body's
immune mechanisms recognize and destroy
genetically mutated cells.
The radiation effects
on the organism occurs mainly under two circumstances :
N When an organism is irradiated with a high dose
of radiation, and too many cells is destroyed,
which the organism is unable to timely replace;
N When the repair
mechanisms at the cell level do not successfully
and correctly repair all the damage and the
body's immune system does not recognize and
eliminate the mutated cells timely, which are then further
divided.
All of these situations, which may
or may not result in biological effects, will be discussed in
more detail below.
Only a small
part of the absorbed energy of radiation is converted into
dissociation and binding energies of chemical reactions, the vast
majority of energy is ultimately converted into heat.
However, at doses used in biological applications, this radiation
heating is negligible.
Hit and radicals mechanism of radiation effect
on cells
Let us now return to the intracellular processes of the radiation
effect, the influence on DNA. Damage to the structure of DNA
occurs through two basic mechanisms :
- Intervention
mechanism
of "direct
effect", according to which damage to an important
part of the cell, especially its nucleus and DNA, occurs
by direct hit with a quantum of
radiation, which involves local energy absorption,
ionization and subsequent chemical change of the affected
structure (Fig.5.2.2a up; in Fig.5.2.1 this corresponds
to the upper branch of the physico-chemical stage).
However, due to the very small size of the target
structures (the DNA double helix
molecule is only about 2 nanometers in diameter), this intervention mechanism is of only secondary
importance, as the probability of such
"direct actions" is relatively low, so that the
sensitivity of living tissue to radiation would be
significantly less than is observed. The direct effect may be somewhat more
pronounced in the case of densely ionizing radiation
(such as alpha, protons).
The actually observed radiosensitivity of living matter
is therefore caused mainly by the second mechanism :
- Radicals
mechanism
of the "indirect effect" is based on the fact that each
organism is composed mainly of water (almost 80%), in which
biologically active substances are dispersed. The
interaction of radiation with living tissue, and thus also with cells, will therefore take place
mainly on water molecules. Due to ionization, radiolysis of water will occur, with the formation of
highly reactive free radicals
H, OH and products capable of oxidation (H2O2, HO2). These
reactive products then attack
the organic molecules of biologically important
substances and chemically them
alter or destroy (Fig.5.2.2a
below; in Fig.5.2.1 this
corresponds to the lower branch of the physico-chemical
stage). The
result can be a number of changes that thus
"indirectly" affect biochemic processes in cells, mainly due to damage to the
structure of DNA. Largest "clusters" of
radicals that are able to disrupt both strands of DNA
have the greatest radiobiological efficacy (see below "Dual Radiation Actions",
"LQ Model"). Even if the primary interaction
of radiation occurs in water outside the cell core, the resulting products can enter
the cell nucleus and exert their harmful
effect there *).
*) For a reflection on the complexity and
diversity of radiation-biological processes, see also the
so-called bystander effect
of remote induction of radiation effects in tissue,
mentioned below (in the section "Effects of
radiation on cells").
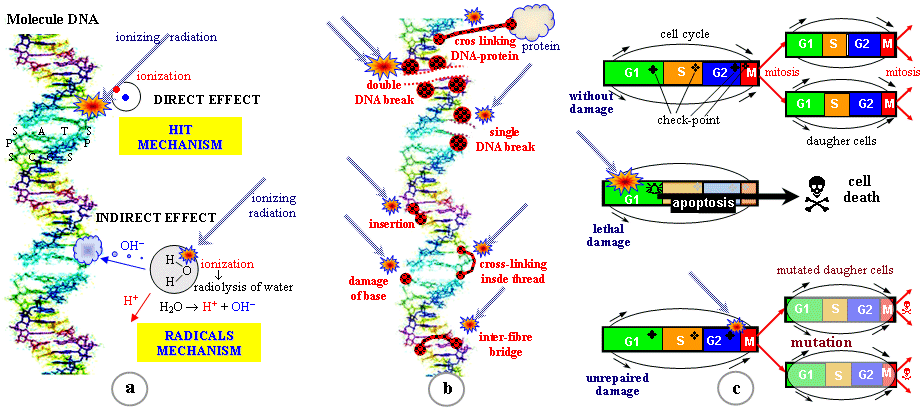
Fig.5.2.2. Radiobiological effects at the subcellular
level.
a) Interventional and radicals mechanism of the effect
of radiation on cell. a) Above:
When radiation enters the DNA macromolecule, ionization and a
direct destructive effect occur. Bottom:
Ionizing radiation interacts with the water molecule,
radiolysising of water occurs: H 2
O ® H +
+ OH - - formation of free
radicals . Highly reactive H + and OH - radicals attack
complex organic molecules and chemically change them
. DNA is disrupted in the nuclei of cells.
b) Different types of DNA damage due to
radiation and chemical influences (rough schematic
drawing). c) Radiation effects during the
cell cycle.
Representation of intervention and
radicals effect
In organisms, complex biochemical molecules are dispersed mainly
in water. In the aqueous medium
(solution, suspension), the proportion of direct and indirect
effect depends on the concentration of molecules
of biologically important substances. When irradiating dried
samples, the direct effect is mainly observed; in the case of
aqueous solutions, the share of the indirect effect is usually
dominant and the higher the lower the concentration. Inside the
cells there is an aqueous environment with a medium-high
concentration of biologically important molecules - the indirect
mechanism is dominant here, but it is necessary to take into
account also the direct effect. The proportion of direct and
indirect effect also depends on whether it is sparsely or densely
ionizing radiation. The radical mechanism of the indirect effect
predominates in sparsely ionizing radiation. Densely ionizing
radiation produces such a high concentration of radicals, that
they often recombine before these radicals can react with
biologically important molecules - somewhat increased importance
can therefore have a direct effect.
Note: In the development of
radiobiological ideas, the effort to explain the effects of
ionizing radiation on living tissue led to the expression of two
basic theories - older interventional and newer radicals
theories. Their role and contribution to radiation effects were
later specified and improved by the mechanism of dual
radiation action and the linear-quadratic model,
discussed below.
Oxygen factor
The effect of ionizing radiation also depends on the presence of
oxygen - the so-called oxygen effect. In the
presence of oxygen, the radiolysis of water produces strongly
oxidizing peroxide radicals, which react irreversibly
with the atoms in the DNA. Thus, the presence of oxygen increases
the radiation effects, especially in sparsely ionizing
radiation. In densely ionizing radiation, where there is an
increased proportion of the direct intervention mechanism (and
also increased radical recombination), the oxygen effect is less
significant. In some cases, the oxygen effect can be
significantly applied in radiotherapy (§3.6, section "Physical
and radiobiological factors of radiotherapy").
Mechanism
of dual radiation action, molecular-biological process
Radical theory was later further improved and refined on the
basis of knowledge of molecular biology. Not
only the total energy (dose) transferred to the
tissue is important for the resulting biological effect, but also
the spatial distribution of this energy in
elementary volumes, as well as the time distribution of the dose.
Microdosimetric analysis of the radiation dose distribution and
monitoring of chromosomal aberrations revealed that the radiation
damage of the cell depends on the ionization density
at the critical site. It turned out that to damage the cell it is
necessary to reach a certain critical value of local energy
density at a given place and time (demonstrated
in 1972 by H.H.Rossi and A.M.Keller). Cell
damage occurs through a combination of two
primary events *) taking place on the double strands of nucleic
acid DNA in the cell nucleus - dual radiation action,
while the probability of damage depends on the number of
fractures and the action of repair processes - it is a complex molecular-biological
process.
*) Disruption of only one strand
(chain) of DNA can usually be easily repaired by the repair
mechanisms of the cell (see below) - this is sublethal
("potentially lethal") damage. Simultaneous damage to
both strands of DNA at nearby sites usually leads to a lethal
effect on the cell. Cellular DNA repair processes do not take
place immediately, but have a certain duration (approximately
tens of minutes). Therefore, at higher intensity (dose rate)
even sparsely ionizing radiation, increases the likelihood that
also a break in the second strand of DNA will occur before the
first strand is repaired, which usually results in lethal cell
damage - individual sublethal damage accumulates in the lethal.
The biological effect then depends not only on the total
radiation dose, but also on its time schedule - the so-called dose
rate effect (discussed below in the LQmodel section ); at higher dose rates,
a greater radiobiological effect occurs.
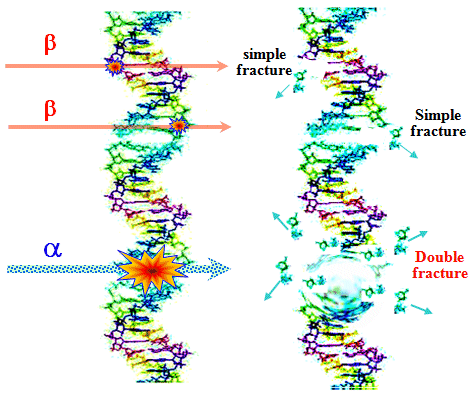 |
Schematic comparison of the effects
of beta and alpha radiation on DNA.
Above:
Beta electrons with a low ionization density cause mostly
simple breaks in DNA that the cell can repair.
Bottom:
Alpha particles with a high ionization density cause
double breaks in DNA, which usually result in cell death
by apoptosis. |
Particles of "sparsely" ionizing
radiation, ie beta and gamma, usually form only one primary
disruption (fracture) during their passage through the critical
site, so that the final occurrence of damage requires the passage
of two individual particles through the site in
rapid succession (so-called b
- process) - the number of these
damages then depends mainly on the square of the dose,
for smaller doses the damage is significantly lower. Particles
"dense" ionizing radiation (alpha, neutrons, protons),
are capable of a single pass through the critical point cause two
or more primary failures (ie. a
-Processes) which is sufficient to
create real damage, so the number of lesions, i.e. radiation
effect, it is directly proportional to the dose radiation;
damage occurs more easily here, these types of radiation have a higher
biological efficiency, which reflects the
above-mentioned quality factor Q. The mathematical
analysis of these processes (a- and b- processes, as well as processes of cell repair and
repopulation) is the so-called linear-quadratic LQ model
of dose-response of the radiation effect; is derived and discussed in detail below - the section
"Dose - Radiation Effect Relationship",
part of LQmodel.
Chromosome aberrations
Because DNA macromolecules are bound in chromosomes, DNA
damage can manifest itself in changes in the shape,
arrangement and structure of chromosomes - so-called chromosomal
aberrations, which can be observed under a microscope. A
number of different chromosome abnormalities have been observed,
which can be divided into two basic groups :
× Numerical aberrations consisting of an
abnormal number of chromosomes whose intrinsic structure is
intact. They arise during cell division, which can lead to errors
in the separation of chromosomes into daughter cells. This can
lead to polyploidy (multiplication of the whole
chromosome set 3 x or 4 × ) or aneuploidy (numerical deviation of only a certain specific
chromosome, which can be multiplied 3 × or
4 ×, or reduced - 1 ×, or completely lost).
× Structural aberrations , where the
structure of a chromosome is disrupted due to chromosome breaks
(caused by double-stranded DNA breaks) and subsequent
rearrangements. Structural aberrations are divided into balanced
(where the amount of original genetic information is
quantitatively preserved) and unbalanced (where part of
the genetic information is missing or in excess). Several types
of changes in chromosome structure are observed:
deletions (part of the chromosome is missing - either
terminal at one end or interstitial in the middle); duplication
of a section of a chromosome; inversion (two breaks on
one chromosome, rewinding a segment between breaks and rejoining
- the order of genetic information changes); translocation
(exchange of two cleaved segments of two chromosomes); fragmentation
(a chromosome splits into two or more smaller parts); annular
chromosome (deletion of the ends of both arms followed by
twisting and joining of the broken ends); - and several others.
Chromosomal aberrations are most
prominently applied until in cell division, where they can either
prevent division or cause uneven distribution of genetic
information into daughter cells. Some chromosomal aberrations are
insignificant and practically harmless, others lead to cell
death, some cause mutations and malformations. Severe and
multiple chromosomal aberrations are observed in tumor cells.
Microscopic (cytogenetic) monitoring of chromosomal aberrations
is also of great importance in the study of the biological
effects of ionizing radiation and in the radiation protection of
exposed persons.
Effects
of radiation on cells
The above-mentioned mechanisms of radiation effects at the
subcellular level result in effects on the basic building blocks
of all living tissues - on cells. When
irradiating a cell with an appropriate dose of radiation, there
are basically two significant types of damage (Fig.5.2.1 on the
right) :
- Cell
death
At very high doses of radiation (hundreds of Gy), due to
the above-mentioned mechanisms, important components of
cell contents are destroyed and denatured,
which lead to immediate cell death even in the
"rest" period, the so-called interphase
(interval between two cell divisions) - cell necrosis
occurs. However, at lower radiation doses, a far more
common type of cell death is the
so-called mitotic cell death, which
occurs during cell division - mitosis. As a result of
irradiation can cause inhibition of cell division. A cell
damaged at some stage of the cell cycle may continue its
metabolism, but is unable to undergo mitosis, which
places high demands on the right conditions for
rearrangement of subcellular structures. Thus, here the
damage does not manifest itself immediately, but only
because the cell is not able to divide further - it
undergoes apoptosis (see "Mechanisms of cell death" below). Mitotic
cell death occurs even at smaller doses (Gy units), which
are not sufficient to induce direct interphase cell
death. It turns out then, that cells that divide
rapidly have higher radiosensitivity
(mitotic death is the predominant
type of cell death due to ionizing radiation).
- Changes
in cell genetic information - mutations
At lower doses of radiation, the cell is not killed
immediately or cell division is not stopped, but the
resulting radicals can cause chemical changes in DNA
(deoxyribonucleic acid) that sometimes fail to repair;
and thus changes in the chromosomes carrying the encoded
genetic information. These changes in cytogenetic
information - mutations - can then be
passed on to other cell generations
during division. According to their extent, mutations are
divided into point or gene and chromosome
ones (chromosome aberrations or changes in
chromosome number). From the point of view of
reproduction, mutations are divided into somatic,
which appear only in a particular irradiated individual
in irradiated tissue (where they can lead to late somatic
damage and tumors - §3.6
"Radiotherapy", passage "Carcinogenesis"), and gametic mutations in
germ cells that can be passed on to future generations in
the offspring of irradiated people (malformations).
Thus, irradiation of cells leads to a number of
harmful changes (radiotoxicity), many
of which may be corrected by the body's repair mechanisms, but
some may lead to cell destruction and some changes (eg in the DNA
code) may be permanent or reproducible. Tissues with intensive
cell division, such as hematopoietic or tumorous, mucous
membranes, developing fetuses (especially in the early stages of
development) are particularly sensitive to the effects of
ionizing radiation.
Radiation
effects during the cell cycle
The effect of radiation on cells depends, in addition to the
physicochemical factors mentioned, on the type of cells and on
the time phase in the cell cycle when the
irradiation occurred.
Cell
division - the cell cycle
The cell cycle is a sequence of
time-ordered processes that a cell goes through in the period
between its divisions - it is the period from
the formation of a cell to the previous division of the mother
cell into two daughters until the stage when it divides
again into two daughter cells (only very
schematically shown in Fig.5.2.2c). Cycle duration (so-called generation
time) is different for various cell types:
for rapidly dividing cells it is only 20-24 hours, for others it
divides only 2 times a year (eg hepatocytes), some do not divide
at all (neurons, eye lens cells). The basic division of the cell
cycle is the "resting" period of interphase G
(gap) between two divisions and phase M
of the cell division itself, mitosis (Greek mitos = thread, fiber). In more detail, the cell cycle of eukaryotic cells is
divided into several phases (Fig.5.2.2c) :
¨ G1 phase - postmitotic
- after the end of previous division, there is a period of cells
growth (G), synthesis of RNA, proteins. Physiological processes
take place in a cell, given the type of a particular cell and its
function in the organism. It takes about 10-12 hours.
¨ G0 phase - rest . During the G1 phase,
the cell can leave this phase (and the whole cell cycle) and
enter the resting so-called G0 phase, where it
no longer divides. This is especially true for fully
differentiated cells such as neurons. Some cells are
able to leave the G0 state, go into the G1 phase and start
dividing again if necessary (eg hepatocytes), while the neurons
remain in the G0 state permanently and no longer divide.
¨ S phase - synthetic -
DNA replicates to double the number (duplication of genetic
material). Duration is about 6-10 hours.
¨ G2
phase - premitotic - duplicates organelles and forms the
structures needed for cell division. It takes about 2-4 hours.
¨ M phase - mitotic - there is nuclear
division (karyokinesis) and then the division of the
whole cell (cytokinesis). The mitotic phase, which lasts
about 1-2 hours, is divided into 5 consecutive stages :
× Prophase - a
preparatory phase of karyogenesis, during which the nuclear
membrane dissolves and chromosomes unfold (chromosomes are
doubled after the S-phase, but are still connected in the centromere
). Cytoplasmic microtubules depolymerize and the released tubulin
is used to polymerize three types of new microtubules in the
nucleus: - astral microtubules, diverging radially from
both centrosomes; - polar microtubules , forming a dividing
spindle between the centrosomes ; - kinetochore
microtubules , involved in the pull of split chromosomes to
the poles of the spindle.
× Metaphase - chromosomes line up in the
equatorial plane of the spindle, there is a pull on opposite
sides to the poles of the spindle.
× Anaphase -
chromosomes rupture into two equal parts by shortening the
microtubules of the dividing spindle.
× Telophase -
the dividing spindle disappears, chromosomes are concentrated in
two new nuclei, around which a nuclear membrane is formed. This
completes karyogenesis.
× Cytokinesis -
a cytoplasmic barrier is formed between the two parts. This
occurs either by strangulation due to the contractile ring
(in animal cells); in yeast it is budding, in plant cells a
compartment between the cells grows from the center to the edge.
The mother cell is thus divided into
two identical daughter cells *), which enter the G1 phase; ®
cycle repeats - if is not stopped by the check
nodes (described below), physiologically mainly restriction G1-checkpoint,
when the cell is put into the "service" G0 phase, it
is not further divided and performs its differentiated function.
Considerably simpler manner undergoing division prokaryotic
cells (bacteria): it is binary division, in
which the cell first elongates into an elongated shape,
replicates its DNA, begins to form a septum in the middle,
whereby the mother cell then splits into two identical daughter
cells.
*) Stem and effector
cells
In multicellular organisms with different differentiated tissues,
the mechanism of division is more complex. Here, the tissues
consist of stem cells (maternal, clonogenic) and
daughter, effector cells. Stem cells are capable
of unlimited division, while they are able to produce both
identical stem cells and daughter cells, bearing the properties
of a given differentiated tissue - asymmetric division
to two different cells, an identical stem cell and a different
daughter cell. Due to their division, clonogenic
stem cells are more radiation-sensitive than
daughter effector cells (which, in addition, are continuously
replenished by stem cell division).
Asymmetric division ensures the process of
differentiation, in which cells, by their
structure and function, adapt to the specific role they are to
play in the organism. All cells in the body are equipped with the
same genetic equipment, but as a result of this process, only a
certain part of the genetic information is realized in a
particular cell (due to specific transcription factors
only certain genes are transcribed in the cell, others are not
applied). Daughter effector cells usually enter the quiescent G0
phase of the cell cycle.
Cell
cycle check nodes
The complex process of cell
division is controlled by several stages of
regulation. The key points of the cell cycle are certain "check
nodes" or points (Check Point), in which
the state of the cell in a given phase and its ability to
continue the cycle is "evaluated" (Fig.5.2.2c). In the
check nodes, it is verified whether all events taking place in
the respective phase of the cell cycle have been successfully
completed - before the cell proceeds to the next stage of its
cycle. The main role of check points is to "supervise"
the correctness or damage of DNA, to ensure proper cell division
with maximum flawless transmission of genetic information.
Certain control mechanisms probably work continuously at every
moment of the cell cycle, but their activity results in several
significant milestones of individual stages :
Y First checkpoint G1 (G1-checkpoint ) or G1/S is at the end of the G1 phase. Here it is
decided whether the cell will continue the division cycle or go
into the resting G0 phase. Control node G1 is sometimes also
called start (only from this point a
new cell cycle can continue) or restriction
- it forms a kind of "barrier" to the continuation of
the cycle, which is overcome by the expression of cyclin D
induced by growth factors; otherwise, the cell remains in the G0
resting phase.
Y The second check node is in S-phase
and controls DNA transcription; suspends the cell cycle until, it
is completely replicated DNA.
Y Third G2/M check
point, located at the end of phase G2 (G2-checkpoint)
controls a number of factors that determine whether a cell is
ready to successfully enter mitosis. It oversees the integrity of
DNA, examines whether DNA is damaged and whether its synthesis
has been completed, delays the onset of mitosis, and stops the
cell cycle in the event of DNA damage.
Y The last control node M (M-checkpoint)
comes into play only during mitosis in anaphase and
checks the correct course and termination of mitosis. The
signaling pathway of the mitotic control node M checks, among
other things, the correct connection of the chromosome
kinetochore to the dividing spindle.
If the control mechanisms find an
inconsistency in one of the control nodes, the cell cycle is
interrupted: either repair processes
are started, or in the case of more severe irreparable damage
(such as a double DNA break), apoptosis may be
initiated (Fig.5.2.2c in the middle) - "programmed"
cell death described in more detail above in section "Cell death".
Radiosensitivity of cells and tissues
The greatest radiosensitivity is manifested when the cell is
affected in the late G1 and premitotic G2 phase, when checkpoint
mechanisms can stop the cell cycle. Slightly lower sensitivity is
in the M phase and in the transition between the G1 and S phases,
the lowest sensitivity for cells in the G0 phase.
Living tissue is made up of a mixture of
cells at various stages of the cell cycle. The average time that
cells are in certain phases depends on the type of tissue.
Tissues growing rapidly with a short cell cycle
have a higher time proportion of G2 and M cell phases, so they
are more sensitive to radiation. Slow-growing
well-differentiated tissues (such as nerve, muscle, ligament) are
relatively radioresistant, as most cells remain
in the G1 phase for a relatively long time and some are even in
the G0 resting phase. Different radiation sensitivities of
individual tissue types are important from the point of view of radiation
protection and play a key role in the radiation
treatment (§3.6 "Radiotherapy" ), where fractional
irradiation and sometimes a combination of chemotherapy and
radiotherapy are used, among other things to achieve some
synchronization of the cell cycle with irradiation cycle.
Radiation damage =>
poisoning of the organism with chemical poison
From the above description of the mechanism of the harmful effect
of ionizing radiation on living tissue, it follows that the
radiation effects are not any "mysterious" unusual
phenomena caused by invisible radiation. The final effect is chemical
or chemical-biological: the radiation only
supplies energy to the tissue (in a specific
form of ionization), which ultimately leads to the production
of "poison" (free radicals)
and internal chemical "poisoning"
of cells - overal we talk about radiotoxicity.
After all, it is similar to diffusingly applying, for example,
hydrogen peroxide or another highly reactive chemical to
a tissue, having denaturing or genotoxic
effects. There are three characteristic differences between
chemical poisoning and radiotoxicity :
1. Chemical poison molecules are delivered to the tissue from
the outside, they penetrate the metabolic pathway
(blood, lymphatic), they accumulate inhomogeneously in certain
cell types. Ionizing radiation supplies only energy and toxic
substances are generated inside cells and
tissues, while they are distributed practically homogeneously
in the entire volume of irradiated tissue, inside all cells.
The situation is more complicated at internal
contamination with a radioactive substance, when its
distribution is inhomogeneous - in the target tissues and organs,
depending on the chemical composition. And also for short-range
radiation in the tissue (a, b), when only the immediate vicinity of the interaction
site is irradiated.
2. Difference in the nature of the dose-response
effect. When lower concentrations of chemical toxicants
are applied, almost all cells survive, and only when a certain
threshold concentration is exceeded will practically all cells in
the population die. When exposed to ionizing radiation, even
small doses can result in the destruction of a small percentage
of cells, but even after receiving very high doses, a certain
part of the cell population survives (see "LQ model"
below). The radiobiological effect has a statistical probabilistic
character, it is governed Poisson distribution *) of
probability. After irradiation of a set of N0 cells, N cells survive, whereas N/N0 ~ e - (number of lethal lesions). This dependence is mathematically realized in a
linear-quadratic model, derived below.
*) The Poisson distribution generally
models random events in a set of a large number of elements in a
situation where the probability of a given process is relatively
small with respect to the total number of elements. If we have a
set of elements (atoms, cells) with instantaneous number N
, in which the given stochastic process (nuclear decay,
cell damage) is caused by the control factor f
(time, dose) with probability l, then the element of the factor Df causes the loss of the
members of the set by DN = - l . Df elements. This leads to a differential equation for
the dependence of the number of remaining elements N on
the acting factor f : dN / df = - l , the solution of which
under the boundary initial conditions N(f = 0) = No is an exponential
function N = No .e - l .
f. It is derived and discussed in
more detail in §1.2, part "General laws of atomic nucleus
transformation" for the case of
radioactive decay.
3. Another difference in DNA damage caused by common
factors (such as oxidants from metabolism) and ionizing radiation
is that after ionizing irradiation there is a increased ossurence
of clusters of DNA damage (especially in densely
ionizing radiation); for common non-radiation damage, this
distribution is more even.
Repair
processes
Cells are not completely defenseless against radiation damage;
when irradiating living tissue, there are not only one-way and
irreversible changes leading to damage to cellular structures and
their functions. In the biological stage of the radiation effect,
there are also opposing processes - processes of repair
and regeneration, which lead to the restoration
of the ability of cell division and function of tissues and
organs. Thus, radiation changes in living tissues may be reversible.
In the early stages
of evolution, cells lived under the influence of shortwave
radiation, especially ultraviolet radiation. During evolution,
mechanisms have had to evolve that maintain the integrity of DNA
(relative stability of genetic information). Enzymatic repair
systems, which are able to eliminate DNA damage (radiation or
chemically induced), have been a positive evolutionary
factor for cells and have mostly been preserved
in cells. It is also worth mentioning the fact that repair
systems are not closely specific to only one harmful
substance.
There are basically two types of repair
processes at two different levels :
- Intracellular
repair
is the ability of a cell to repair its
vital structures, damaged by ionizing radiation or other
influences (and in good time, before further damage
occurs, which could make repair impossible). At the level
of the affected cell, antioxidants
act protective against reactive radicals, as well as
enzymatic processes occurs, which repair
damaged DNA structures and processes that remove those of
them, that can no longer be repaired. Due to these
chromosomal repair mechanisms, the cell
can regain its function and ability
to divide within a few hours after irradiation.
Repair mechanisms at the molecular
intracellular level consist of biochemical
processes that lead to the repair of
certain types of DNA damage. DNA damage activates ATM
kinase, which triggers the production of
DNA repair proteins - DNA ligase - and
(via mdm2) activates and phosphorylates the p53
protein, which acts as a transcription factor. In the
early stages, p53 can activate DNA repair (via GADD45
proteins) and slow down the cell cycle (via p21
protein encoded by the
WAF/CIP1 gene), so that there is enough time for the
repair to take place. The p21 protein inhibits cyclin-dependent
CDK kinases and arrests the cell cycle in the G1
phase; in the case of proper function of p53, the cell
cycle is restored only after the elimination of errors in
DNA. If p53 detects DNA damage beyond repair, its
expression is increased and the process of cell apoptosis
described above is initiated ("Mechanisms of Cell Death").
Repair processes are associated
with the activity of a number of (repair) enzymes,
called endonucleases, exonucleases, polymerases,
ligases. For example, the nibrin protein is
used to repair chromosomal breaks. An important
controlling role in nitrocellular reparations is played
by the p53 protein, the "genetic
guardian" mentioned above in the "Mechanisms of Cell Death", "Apoptosis" section.
This p53 looks for DNA damaged sites *):
if it finds such a site, it binds to them and initiates
the production of enzymes for DNA repair. At the same
time, it triggers the expression of the p21
protein, which stops the cell cycle until the damaged
site is repaired. If repair is unsuccessful, cell
apoptosis eventually occurs.
*) On "search"
for damaged DNA sites contributes the histone
H2AX protein, around which DNA is
"wound" in chromosomes. Immediately after DNA
damage, phosphorylation occurs at the sites of
damage, in which a phosphate group binds to H2AX. This
"marks" the sites that need to be repaired -
into the phosphorylated portion of H2AX a certain part of
the binding protein 53BP1 for p53 fits ("as a lock
key"), whereby starts the process of repairing the
broken DNA strand.
¨ Simple DNA
breaks can be repaired relatively easily by
so-called excision repair: the
damaged section of the DNA strand is "cut out"
by the enzymatic action of endonucleases, the gap created
is then the enzyme DNA-ligase replaces with a
"patch" synthesized based on a second
"sister" string whose continuity has been
preserved - this string is used as a template to
repair a damaged string.
¨
It is difficult for the cell to repair double
DNA breaks, when part of the information on both
strands is lost *). These breaks can be successfully
repaired under certain circumstances by certain reciprocal
recombination processes, where the damaged sites
(again with the participation of enzymes) recombine with
each other with corresponding intact sections of the
"sister" strand, which act as a template for
repairing the excised site of the damaged DNA strand. Or,
damaged sections may be "ignored" and
"provisionally" joined to maintain DNA
integrity.
*) It also depends on
the structure of the chromosomal system of the cell. In haploid
cells (with one set of chromosomes), individual double
DNA breaks already lead to their inactivation. However,
most somatic cells are diploid - the chromosomal
system is doubled in them, so that individual double DNA
breaks can often be repaired. Cell death is usually
caused by simultaneous damage to a pair of homologous
chromosomes, or a number of double breaks at different
sites in the chromosomal system.
Thus, two repair mechanisms are
known here :
- Homologous recombination
, in which the repair is performed by exchanging the same
or similar (homologous) DNA sequence as that
which was damaged. It takes place after the S-phase, when
the DNA is already doubled and it is therefore possible
to find the required section in the "sister"
DNA as a template. According to this complementary
strand, the disrupted region of the DNA molecule is
synthesized; the result is usually an accurate correction
without changing the genetic information. However,
finding the corresponding homologous sequences in a
complex chain of DNA is difficult, so that more common
and simpler mechanism is a direct coupling :
- Nonhomologous bonding
, the non-homologous end-joining (NHEJ),
in which broken DNA strand are directly connected at
their ends, without the use of homologous template. This
method is faster and easier, but may be a source of errors
in the DNA sequence - changes in genetic information. The integrity of the DNA and the viability of
the cell can thus be restored; with "a little
luck" the damaged and lost section did not encode
anything important (only about
5-10% of the genome encodes functional proteins, most of
the DNA sequences are a kind of "genetic
garbage" which is relic of the intricate path of
evolution) ...
The most difficult is the repair of damage of both chains
in the same section, or in not too distant places.
The success of
the repair depends also on the dose rate. The
higher the dose rate, the more likely it is that even a
break in the second strand of DNA will occur before the
first strand has been repaired. In this case, lethal
cell damage usually occurs, and repair is unsuccessful.
Faulty
mutagenic repairs
However, repair processes, in the one hand, may not be
successful and, on the other hand, may lead to new
mutation-inducing errors. During the operation of repair
mechanisms, there is a certain probability that the wrong
nucleotide, or series of nucleotides will be inserted
into the DNA strand at the site of damage. The cell tries
to maintain the integrity of the DNA (and thus its
viability) even at the cost of imperfect (erroneous)
repairs. This so-called mutagenic
repair sometimes allows the cell to survive, but
from the point of view of the whole multicellular
organism there is a risk of further division of mutated
cells - the emergence of stochastic effects,
especially the risk of tumor growth. From the point of
view of the whole organism, the motto "dead cell
= good cell " applies to radiation-damaged
cells.
- Regeneration
in cell populations
At the level of the affected tissue, the
repair of radiation damage is carried out by replacing
the destroyed cells by dividing the surviving
(stem clonogenic) cells, which have retained their normal
ability to divide - by repopulating
them; this process takes days to weeks. In some cases,
the destroyed tissue is replaced by an non-functional binder.
Probability of biological effect
After a specific "radiation action" (cell intervention
by a quantum of ionizing radiation), the probability of a
biological effect depends mainly on two circumstances :
1. Type of DNA damage
(Fig.5.2.2b). 2. The phase
of the cell cycle in which the damage occurred
(Fig.5.2.2c).
Simple breaks of DNA strands the repair mechanisms can usually
repair flawlessly (if they have "enough time" to do
so). Double DNA breaks, if they occur in the G1 and G2 phases,
are recognized in the "check points" and usually lead
to cell death (Fig.5.2.2c in the middle). However, DNA damage
that occurs after the "checkpoints" stage may not be
recognized by cellular regulatory mechanisms and mitosis may
occur with this error - there is an increased risk of dividing
cells with altered genomes, which may result in mutations
(stochastic effects) - Fig.5.2.2c at the bottom. During the
practical irradiation of the organism, the individual cells in
the tissues are in various phases of their cell cycle and at the
same time DNA of various kinds is damaged. Therefore, individual
microscopic events are averaged into the resulting
radiobiological effect - deterministic or stochastic.
Much depends also on the speed of the cell
cycle. Cells that divide rapidly have, on average, less time to
repair damage to their DNA, so they will suffer more from the
consequences of unrepaired or incorrectly repaired damage. This
leads to an important general conclusion: rapidly
dividing cells are more radiosensitive.
The repair and regeneration processes
lead, inter alia, that the radiation dose dividing into smaller
sub-doses at sufficient time intervals, cause to smaller
biological effects, compared to the same dose absorbed at once.
Respectively, at the same total absorbed dose, the biological
effect reduces if it was achieved at a lower dose rate (which of
course corresponds to a longer exposure time) - see "LQ
model" below - dose rate effect.
Effects of radiation at the tissue level
The effects of radiation at the subcellular and cellular levels
discussed above are the basic starting point for
understanding the effects at higher levels of the organization -
in tissues, organs, the whole body. However, they are not a
completely sufficient starting point! There is a well-known
general experience, that a system is not just a simple sum of
its elements (or "an organized
whole is more than the sum of its parts"). Some radiation effects in living tissue are caused not
only by basic cellular mechanisms, but also by the interaction
of various tissue factors, such as intracellular
communication trought biochemical signaling pathways,
movement of molecules and ions through the intercellular space,
cell division and tissue regeneration. At the level of organs and
the whole organism, complex biological relationships between
different types of cells and tissues in the activity of organs
and functional relationships between individual organs in the
body approach this.
Biochemical
interactions of cells. Extra-target remote-induced radiation
effects - bystander effect, abscopic effect
The view of classical radiobiology
is largely mechanistic: damage occurs only
in cells that are directly affected by ionizing radiation or a
chemical agent, which damages the DNA or other important
structure of the cell. This is indeed the case
when irradiating a set of isolated cells (such
as bacterial colonies). However, in higher organisms, cells are
incorporated into tissues in which cells
biochemically interact with each other through a
number of complex processes (especially regulatory ones). Some of
these processes (repopulation, redistribution, reoxygenation)
are mentioned below in connection with the dependence of
biological effects on the radiation dose and its time course. Due
to these cell interactions, it can be expected that other
surrounding cells in the tissue may react to
(radiation) damage to one particular cell in a certain way.
This process has actually been observed in
experiments with very narrow sharply collimated beams (micro-beam)
radiation a which irradiated cells in
tissue culture. It was found that after irradiation of the target
cells, some surrounding cells showed signs of damage,
even though they were not irradiated themselves.
Experiments in which a sample of irradiated cells was transferred
to a colony of unirradiated cells led to similar results. This
remarkable effect could be due to the mentioned biochemical
interactions of cells in the tissue. The exact mechanism of this
phenomenon is not yet known. The surrounding cells could be
affected in basically three ways :
× Through the intercellular environment
Cells affected by radiation can produce certain diffusible
substances that are able to affect other neighboring cells.
Primary irradiated and lethally damaged cells can "send
chemical signals" to their surroundings (extracellular
matrix) - release biochemical molecules involved in
apoptosis of irreversibly damaged irradiated cells (p53 protein
or TRAIL ligand is considered, as mentioned in the passage above
"Apoptosis"), or molecules of toxic, oxygen or nitrogen
radicals. These substances can diffuse and enter surrounding
cells (or bind to appropriate receptors on the surface, activate
external apoptosis signaling pathways), in which they can cause a
similar response to that of a directly affected cell.
× Over intercellular junction
Intercellular communication (gap
junction) they are carried out by
membrane channels, called connexons, with a diameter of
about 1.5-2 namometers, through which molecules up to 2 kDa can
pass. When one cell is damaged, the canal usually closes quickly,
preventing damage to neighboring cells. However, if this pathway
is not inhibited in time, damage can be transmitted to
surrounding cells.
× Macrophages
Finally, the activity of macrophages could contribute to
the apoptosis of the surrounding unaffected cells. In addition to
the apoptotic cell itself, the attracted activated macrophages
could attack and eat even some neighboring cells.
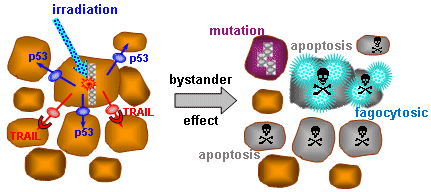 |
Bystander effect.
Radiation damage to a single cell can induce damage to
some surrounding cells, that have not been irradiated. |
For this phenomenon of radiation effects induced
in neighboring cells, is used the name bystander
effect (Eng. Bystander =
viewer, bystanders, gaper) - "effect
of the non-participating spectator":
the surrounding directly unaffected cells are not a "neutral
observers" radiation damage of irradiated cells, but are
also "drawn" into this process. The bystander effect
thus slightly increases the total number of
radiation-damaged cells in the irradiated tissue (Fig.5.2.4b).
The bystander effect can cause apoptosis, chromosomal
aberrations and mutations in
surrounding cells.
Note: The
bystander effect was observed not only in radiation-damaged
cells, but also in local chemical cytotoxic damage.
Abscopal effect
The phenomenon of biological changes in the non-irradiated part
of the biological system as a result of the reaction of the
irradiated part is generally referred to as a side,
extra-target or abscopic effect (lat. Ab = outside, away; scopium = target, aim,
angle of view). Post-radiation
changes usually occur in cells and tissues closely adjacent to
irradiated areas - such as the aforementioned bystander
effect. However, "long-distance" abscopic
effects were also observed. In radiotherapy with local
irradiation of a single tumor lesion sometimes (unfortunately rarely) an immunoeffect
- immunogenic cell death - is also induced against other
metastases of the same tumor (see also
§3.6, section "Immunotherapy"). The exact mechanism of
the aboscopic immunoefect is not yet known, but it is probably
due to massive apoptosis or necrotic death of tumor cells
irradiated with a high radiation dose. This elicits a local
inflammatory immune response in which T
cells form within the irradiated tumor, which can be specifically
activated by the uptake of antigens from tumor cells.
These specifically activated effector T cells then migrate
to unirradiated secondary tumors, where they initiate a targeted
"destructive" immune response against tumor cells of
given type, that have the same antigens (involving monocytes
transforming into macrophages).
In
addition to this highly desirable effect, an adverse abscopal
effect in normal healthy tissues that could induce genomic
instability, cell death, or oncogenic cell transformation is
sometimes discussed. However, such a mechanism has not been
demonstrated, the causes of the side effects of radiation on
healthy tissue are probably different (§3.6, section "Side effects of radiotherapy -
radiotoxicity, secondary malignancy").
It would be unlikely if the radiation-mutated cells merely
migrated and established a distant tumor site without such a site
forming near the irradiated site.
However, a situation could arise where the irradiation
causes a decrease in the body's immunity, as a result of which a
still "dormant" clone of mutated cells may initiate
tumor growth at some point. Such a complex process, however,
probably not be considered as a radiation abscopic effect ..?..
Various
radiosensitivity of cells and tissues
The organism is a functional complex of tissues and organs, that
do not have the same sensitivity to irradiation
(radiosensitivity). At the same absorbed dose, different
biological effects are seen in different tissues. In analyzing
the effects of radiation on cells, we found that rapidly dividing
cells are more sensitive to radiation damage. This basic finding
is also manifested at the tissue level and is sometimes expressed
by the rule: "the radiosensitivity of the tissue is
directly proportional to the reproductive activity and indirectly
proportional to the degree of differentiation" *).
This rule is only approximate, for specific tissues and organs
they are affected by specific biological influences (see below "Local tissue and organ radiation effects"). Tissues and organs have
different sensitivities not only to damage by cell death, but
also different susceptibility to application of cytogenetic
effects, the risk of tumor formation.
*) The direct and inverse
proportionality there cannot be taken mathematically, but only as
an expression of the increasing or decreasing trend. It is
sometimes called the Bergonia-Tribondeau rule, according
to the first authors who came to him empirically.
Relationship
between radiation dose and biological effect
It is obvious, that the biological effect of radiation is
primarily dependent on the size of the absorbed dose - increases
with dose. In terms of dose-effect relationship, we distinguish
between two basic types of radiobiological effects :
- Stochastic
effects - mitogenic
If the radiation dose is not large, with the vast
majority of damage to biologically active substances, the
organism will successfully cope with its repair
mechanisms. However, even with small doses *)
there is a certain probability that some damage will not
be repaired (or an
"error" will occur during repair), there will be post-radiation gene
instability, which may result in mutations.
If the mutated cells further divides,
late permanent consequences of a genetic or tumor nature
may occur. Because such consequences are completely
random, individual, and unpredictable, they are called stochastic
effects. They have a probabilistic character - in
individuals from an irradiated group of persons, the
damage or disease occurs randomly with a
certain probability that increases with the dose. For the
stochastic effects are responsible cells that survive
radiation damage, but the radiation has caused genetic
changes in their DNA.
*) Of course, stochastic effects
(with an even higher probability) can occur even at
higher doses, after eventual unwinding of deterministic
effects.
For stochastic effects, the
severity of the disability and the course of the resulting
disease do not depend on the dose; only the probability
of occurence the tumor or genetic damage depends on the
absorbed dose. These are disease states which, even without the
influence of radiation, occur "spontaneously",
without an obvious cause *), in the population. In individual
cases, it is not possible to distinguish radiation-induced tumors
and genetic changes from spontaneous cases, their clinical
picture is the same (there
are no symptoms specific for tumors caused by ionizing radiation). Ionizing radiation only increases the
likelihood of these diseases, the corresponding risk is additional
to other risks. The average risk factor for
radiation-induced malignancy is estimated at 0.055 Sv-1, or 5.5% per 1Sv (ie if 1000 people receive an effective dose of 1Sv, 55
of them can be expected to cause fatal tumor).
*) As discussed above, this is
probably due to the genotoxicity of some chemicals,
which either enter the cells from the outside (through the body's
metabolic pathway) or are formed inside the cells (during the
internal metabolism of the cells). These substances enter the
nucleus, react with DNA and cause disorders of its gene sequence,
which may not be successfully repaired ...
Dependence
of stochastic effects on age
At the same radiation dose, the probability
of stochastic effects is inversely related to the age
of the irradiated individual. This is due to two circumstances :
¨ Time factor - stochastic radiation
effects have a long latency, the probability of their
manifestation increases with time since irradiation. When the
body is irradiated at a younger age, there is probably more time
available for late stochastic effects to occur. When irradiated
in old age, stochastic effects are often not enough to apply
until the end of life.
¨ In
children, due to growth, there is a more intense cell
division, which leads to higher radiosensitivity.
Linear-quadratic dose dependence of stochastic effects
According to the theory of dual radiation action,
the probability of radiation damage to the cell - and thus the
probability of unsuccessful DNA repair ® the probability of
mutations and the occurrence of stochastic effects, especially
malignant transformations, depends on the ionization
density at a given site. Similar to deterministic
effects (where radiation cell killing has a linear-quadratic dose
dependence - see the " LQ model " for derivation
below ), so for stochastic effects, the probability distribution
of malignant transformations at an effective dose is linear-quadratic.
At low doses up to about 1Sv, the curve is linear the
shape is directly proportional to the frequency of malignancies
at the dose (black line in Fig.5.2.3a), at higher doses the
occurrence of radiation-induced malignancies is proportional to
the square of the effective dose - quadratic dependence.
However, the theoretically expected quadratic dependence of
stochastic effects for higher doses is difficult to demonstrate,
given that at high doses almost all cells in the population die
rapidly with deterministic effects.
Linear threshold-free dependence of
stochastic effects (?)
Thus, at low doses, for stochastic effects, it is usually assumed
that the degree of effect, ie the probability of
radiation-induced damage (tumor or genetic)
is linearly dose-dependent
(black line in Fig.5.2.3a) and that the stochastic effects are non-threshold
- they can be caused even by very small doses, even at the level
of the natural radiation background, although with a slight
probability. In classical radiobiology, it is hypothetically
assumed that each mutation of an individual cell
(whether generated by radiation or
biochemically) can be the first step to carcinogenesis
(it is discussed in §3.6 "Radiotherapy"). Even if this were,
however, the mutagenic effects of very low radiation doses may be
questionable..?.. - will be discussed below.
Note: In
the interest of scientific objectivity, it would
perhaps be appropriate to point out at this point that in the
area of very low doses <0.2 Gy, the generally accepted linear
threshold-free dependence is only a hypothesis,
resulting from the direct extrapolation of proven effects from
higher dose areas (> 0.5Gy) towards zero, to the low dose
range, where these effects have never been directly demonstrated
(discussed in more detail below in the
section "Small doses of radiation: - are
harmful or beneficial?").
The "conservative
approach" to radiation protection is based on this
assumption, the so-called linear threshold-free
dependence of stochastic effects, which is reflected in
a number of standards and regulations for working with ionizing
radiation. However, recent radiobiological studies indicate that
the dose-response of stochastic effects is not linear, but that
at very low doses the effects are probably lower
than would correspond to the linear dependence *) - blue curve in
Fig.5.2.3a.
*) This behavior could be
related to radiation-induced repair and also to
the so-called hyper-radiosensitivity to low doses (see below "Deviations from the LQ model"), when irradiated cells (with the risk of altered
genome) with increased more likelyhood disappear. The views of
radiobiologists differ here, and the relatively increased
probability of low-dose stochastic effects is sometimes
discussed, eg in connection with the afore mentioned bystander
effect ..?..
In addition, if some alternative
views are confirmed (mentioned
below "Small doses of radiation:
- are they harmful or beneficial?"), the curve of the
dependence of stochastic effects on the radiation dose could in
fact be in the form of a green curve in Fig.5.2.3a - even for
stochastic effects could be exist a threshold
(!) (similar to deterministic effects), but many times lower. And at the lowest doses, the
so-called radiation hormesis could manifest
itself - a section of the green curve of the curve below the
horizontal axis..?.. Radiobiological results will certainly be
refined, but for small doses of radiation, comparable to the
natural level to which our organisms are "adapted", at
present there is no direct evidence that low
levels of radiation are harmful to health (see
the discussion below in the section "Small doses of radiation: - are
they harmful or beneficial?").
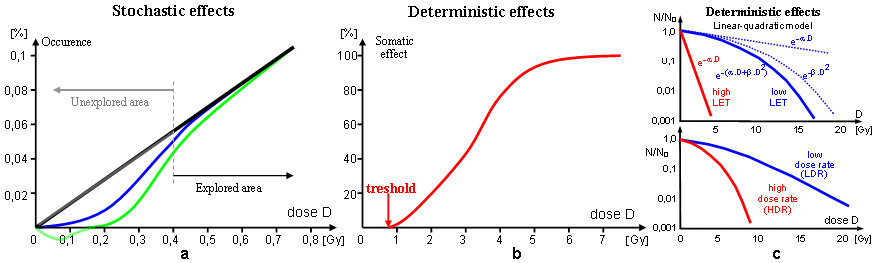
Fig.5.2.3. Dependence of biological effect
on the size of absorbed radiation dose.
a) Probability of occurrence for stochastic
effects. b) Severity of damage for deterministic
effects.
c) Dose dependences of the surviving fraction of N/N0 cells according to a
linear-quadratic model.
- Deterministic
effects - tissue reactions
At high doses of radiation, the number of damaged
molecules of biologically active substances is already so
high, that neither the cells nor the organism are able to
repair them completely - a large part of the
cells die, radiation disease arises.
The tissue damage here is directly proportional to the
received radiation dose, it is no longer accidental, on
the contrary it is predictable *) - we
are talking about the effects of deterministic - tissue
and organ reactions.
*) However, it is useful to recall,
that even in the essence of deterministic effects lies
the stochastic mechanism of cell death (see
below "LQ model ").
However, at higher doses, the number of damaged cells is
so high, that the statistical character is no
longer manifested (the relative scattering S = 1/ÖN of the
number N of damaged cells is very small).
Deterministic effects become
clinically manifest until after a certain threshold dose
is reached, while with increasing dose increases, the probability
of damage (i.e. when irradiated group of
people, the number of individuals who show damage increases; at
higher doses the effects are seen in everyone) and mainly the severity of the damage increases
with the idividual's dose. The dependence of the radiation effect
on the absorbed dose for deterministic effects is shown in
Fig.5.2.3 in the middle. The basic pathogenic mechanism is a reduction
in the number of cells (cells depletion) in the
irradiated tissue. Toxic substances also contribute to
the harmful effect, arising from the extinction and decomposition
of a large number of cells. The sigmoidal shape of the curve,
beginning from a certain dose threshold, is a reflection of the
fact that there is a certain functional reserve,
usually quite large, in the irradiated tissue (cell population).
Therefore, a decrease in the number of cells with increasing dose
does not initially cause any functional problems in the
irradiated tissue, only at higher doses does the deficit
of cells lead to somatic manifestations. The value of
the threshold dose for humans around 1Gy
according to Fig.5.2.3 b is only average (whole
body) and indicative. Each tissue generally has a different
threshold dose of deterministic effects, depending on cell
radiosensitivity and functional reserve in the
tissue - eg approximately: skin 3Gy, lungs 5Gy, sperm 0.3Gy, eye
lens 1.5Gy, developing embryo in utero 0.1Gy, .....
Pathologically
increased radiosensitivity
occurs in individuals with genetic disorder of chromosomal
instability, where the ability of repair mechanisms
at the cellular level is reduced (impairment of stability and
reparability of DNA by homologous recombination -
hypersensitivity to genotoxic effects), as well as reduction of
immune processes (immunosuppression) at the organism level *). If
both dominant genes are in order in a heterozygous cell,
radiation or other damage to one of them may not impair cell
function. However, if one gene is OK and the other is disrupted,
damage to one gene can lead to total cell dysfunction. In homozygotes
with such a reparability disorder, the consequences of the damage
are even more pronounced, they are fully applied. In these
individuals, the threshold dose is reduced for
deterministic effects, and even diagnostic irradiation (eg more
demanding X-ray examination of CT or angio, dose approx. 40-60
mSv) can cause slight deterministic effects in them.
During radiotherapy is manifest a increased radiotoxicity
to healthy tissues, in chemotherapy increased sensitivity to cytostatics.
Also, the risk of stochastic effects, even from the
natural radiation background or other pollutants (chemical,
including metabolic products), is significantly higher - more
frequent occurrence of tumors (especially lymphomas).
*) One of such disorders is the so-called Nijmegen
breakage syndrome (NBS, the name is derived from a city in
the Netherlands, where the disorder was first identified in 1981
and where there is a central registry of these patients) -
autosomal inherited syndrome of chromosomal instability. It is
caused by a mutation in a gene called NBS1 or NBN,
which encodes the protein nibrin, which has an important
function in repairing chromosomal breaks caused by ionizing
radiation or other genotoxic agents. Genetic chromosomal
instability also occurs in Ataxia telangiectasia
syndromes, Bloom's syndrome, Fanconi anemia,
Xeroderma pigmentosum, Li-Fraumeni syndrome mutated
TP53 gene encoding p53, or the very rare Werner
premature aging syndrome. A number of pathological
clinical manifestations result from cell cycle disorders. In
addition to increased radiosensitivity and more frequent
occurrence of cancer, there are also growth disorders,
neurological and skin manifestations, and infectious diseases
often occur due to reduced immunity. Fortunately, these
congenital inherited disorders, which are not causally treatable
(only their specific clinical manifestations can be treated),
occur very rarely, the total incidence of all mentioned species
is about 0.02% of the population. However, heterozygous carriers
is significantly more, about 0.5%.
Note: Please, do
not confuse this pathologically increased overall
radiosensitivity with physiological hyper-radiosensitivity
(relative) in the area of low radiation doses, which is inherent
in all cells - is discussed below in the section "Hyperradiosensitivity
to low doses. Model of induced repair".
Deterministic
+ stochastic effects
The stochastic and deterministic
effects of radiation have basic intracellular mechanisms and some
external aspects in common and cannot always be
strictly separated from each other. The basis of deterministic
effects lies in the mechanism of radiation killing of
cells, which has a probabilistic (ie
"stochastic") character at the cellular level, is
governed by Poisson statistics (see below "LQ
model"). At medium and higher doses, however, the
number of killed cells is so large, that statistical fluctuations
are practically do not manifested, the resulting dependence is deterministic.
Even at low doses (below the threshold of deterministic effects),
for which only stochastic effects are usually discussed, a small
number of cells are "covertly" killed; externally, this
does not manifest itself simply because the remaining intact
cells are sufficient to provide a functional need for tissue or
organ, and are replaced in time by the division of these intact
cells. The LQ model not only describes deterministic
radiobiological effects, but also implicitly lies in the
foundations of probability of stochastic effects.
It should also be borne in mind that, along with the early
deterministic effects are may latently apply even late
stochastic effects, if the organism survives the
deterministic effects. This is observed with radiotherapy based
on deterministic effects on tumor tissue, where in addition to
acute radiotoxicity, secondary post -radiation malignancies
may occur over time, due to the stochastic effects of the part of
the radiation absorbed outside the primary target tumor
(including scattered radiation) and irradiated even the other
tissues and organs (§3.6 "Radiotherapy", part "Physical and
radiobiological factors of radiotherapy").
Terminological note:
In our text, we often use the term "deterministic
radiation effect" in a somewhat weaker and more general
sense than in radiation protection - in the sense of lethal
damage and cell death, without the need for manifestation of
somatic effects for the whole organism. Thus, "deterministic
effects at the cellular and local tissue and organ level"
(eg in radiotherapy).
| Comparison
of the nature of deterministic and stochastic effects of
radiation on the organism |
| Properties: |
Deterministic
effects |
Stochastic
effects |
| Pathogenesis: |
Cell death - reducing their number |
Change of cytogenetic information -
mutations |
| Specificity: |
Specific clinical picture,
typical for the effects of ionizing radiation |
Non-specific image,
indistinguishable from spontaneous cases |
| Dose dependence: |
The effect only becomes apparent from
a certain
threshold dose, then increases with dose |
The probability of occurrence
increases
with dose from zero (threshold-free dependence) |
| Time dependence: |
Mostly relatively fast onset |
Late effects, long latency |
Radiobiological modeling
The radiobiological
effects of radiation on cells and tissues generally
depend mainly on the absorbed dose, on the type of radiation, the
type of cells in the tissue and also on time factors. These
generally complex dependencies have been measured in a number of
radiobiological experiments and verified in clinical studies,
especially in radiotherapy. There was a natural attempt to
describe these empirical laws using mathematical models
and functions, that would express the dependence of the
radiobiological response on dose D , its time distribution
and the properties of irradiated tissue. Models that would allow
to compare and analyze different radiobiological data and predict
cell survival based on physical aspects (type
and energy of quantum radiation) and biological
properties of cells and tissues (radiosensitivity,
repair capabilities of normal and tumor cell lines).
It is mainly a functional expression
of the surviving number of N cells from the originally
irradiated number N0, resp. dose dependence of the surviving cell
fraction [N/N0] (D). Until to the 70s, this modeling consisted in the
construction of empirical mathematical functions
containing appropriate powers of dose D , irradiation time
T and number of fractions n (powers
were mostly non-integer, eg T0.33 or T0.11,
n0.24 etc. - so did eg. M.Standquist in 1946, or
F.Ellis in 1969); these functions were
interleaved (fit) from empirically determined dependencies.
A major advance in radiobiological
modeling occurred in the 1980s, when a so-called linear-quadratic
(LQ) model was formulated on the basis of more detailed
microdosimetric measurements and evaluation of chromosomal
aberrations - see below. This approach is no longer purely
empirical, but is based on an analysis of the mechanisms
of the process of damage and killing of cells by ionizing
radiation at the subcellular and molecular level. The parameters
of this model (coefficients a,
b and time factors) can be derived from the
measured data (experimental and clinical), which allows within one
model distinguish the behavior and responses of
different tissue types. The LQ model is used primarily for
deterministic radiation effects, but implicitly also lies in the
basis of stochastic effects. We will analyze the LQ model in more
detail here, then briefly list some other modified models below.
Linear-quadratic
(LQ) model of radiobiological effect
The deterministic radiation effect, consisting in the destruction
of a larger number of cells, is caused mainly breakage of
both strands of DNA in the nuclei of cells. As discussed
above (see "Intervention and
Radicals Theory of Radiation Effect"), according to the theory of dual radiation action,
radiation damage to a cell depends on the ionization
density at a critical site. To determine the functional
expression of the surviving number of N cells (from the
originally irradiated number of N0 cells) on the received dose D , the LQ model
uses three initial assumptions :
× The
break of a single strand of DNA can be easily repaired -
sublethal damage ® cell survival.
× The break of both chains (strands) of DNA is difficult
to repair - lethal damage ® usually cell death (apoptosis).
× It is probabilistic events with
Poisson statistics: after irradiation of a set
of N0
cells, N cells survive, given by the exponential
regularity N = N0 .e- <probability of lethal cell damage>.

Double DNA break can be caused by two types of
processes :
¨ a -process - intervention of one ionizing
particle, which breaks both strands-chains of DNA at the same
time (manifests itself mainly in densely ionizing radiation, but
also in sparse ionizing radiation secondary electrons can damage
both branches of DNA). The number of irreversibly damaged cells
is directly proportional to the dose - a linear
dependence on the dose D. If the initial number of cells
is N0,
then after irradiation the number of surviving cells can be
expressed by the exponential relationship N = N0 .e -a .D, where a is average probability of a-damage per unit dose (derivation from Poisson's statistical distribution is
analogous to the exponential law of radioactive decay - see
§2.2, section "General laws of atomic nucleus
transformation", instead
of time t would be dose D ).
Coefficient values a range from about 0.1 ¸
0.8 Gy-1.
¨ b -process - time-close interventions of
two independent ionizing quanta, in which each of them breaks one
strand of DNA (breaking one strand of DNA allows repair - sublethal
damage, damage to both strands is usually lethal). The
number of radiation-damaged cells here is proportional to the
square of the dose - quadratic dependence on
dose D. The relevant exponential law of cell number decrease in
this case will be N = N0 .e -b .D2, where b is the average probability of b-damage per square unit
dose. The dependence is initially gradual, almost linear
(possibility of cell repair after sublethal damage), then turns
into a steeper exponential course, caused by the accumulation of
sublethal effects in lethal. The coefficient b acquires values of
about 0.01 ¸ 0.1 Gy-2 for human cells.
The total probability of cell survival when applying
both processes will then be given by the product of individual
probabilities, which leads to the resulting exponential law:
N = N0 .e- (a .D
+ b .D2). The dose dependence of cell survival is often
expressed by the curve of the surviving fraction
of cells N/N0 on a (semi) logarithmic scale :
- ln (N/N0) = a .D + b .D2 - linear-quadratic
dependence, Fig.5.2.3c above.
For densely ionizing radiation (high LET, mainly a-process) this
curve has an almost linear shape, for sparsely
ionizing radiation (low LET, b-process predominates) the
graph has the shape of a parabola.
In applications of LQ model, an a/b
ratio [Gy] is often introduced to express the
dose, at which the damage by the a-mechanism is the same as
the b-mechanism
(the linear and quadratic components are biologically equivalent
in terms of the resulting lethal effect for cells). Graphically,
the value of the ratio a/b characterizes the curvature of the graph of the
dependence of the surviving fraction of cells ln(N/N0) on the dose
according to Fig.5.2.3c. Values a/b they depend on the
relative representation of the individual phases of the cell
cycle. For rapidly dividing cells (eg tumor cells) the values of
the ratio are a/b » 10 Gy, for normal (late-reacting) tissues is a/b » 2 ¸ 4 Gy. The a/b ratio appears in
the derived biophysical dose quantity, which is the so-called biologically
effective dose (biological equivalent of dose)
BED = -ln(N/N0)/a = D.[1 + D/(a/b)]. BED is important in radiotherapy in
assessiment the effect of fractionation of the total
radiation dose D on n sub fractions d , when
BED = D.[1 + d/(a/b)] (see §3.6 "Radiotherapy", section
"Physical and radiobiological
factors of radiotherapy")
.
Time factor
Such simple dependences apply in the case
of a single exposure. In the case of time-prolonged
irradiation - continuous or repeated (fractionated), in
addition to the mentioned mechanisms, time factors of
cell repair (especially in the case of b-damage) and repopulation
of cells by division in tissue, also apply. When
irradiated with dose D during the irradiation time T in the LQ model, two additional
quantities are introduced into the initial linear-quadratic
dependence - factors of cell regeneration RG and
repopulation RP :
1. Reparation
For each elementary time interval Dt, during which the cells
receive a dose DD = D. Dt /T and the N. b. DD2
cells are damaged, at the same time it is also enough to
regenerate the N. l. Dt cells, where the parameter l is the rate of
cell repair (l = ln2 /T1/2, where T1/2 is the half-life of repair). Integration from
0 to T gives a modified exponential law N = N0 .e- RG.
b .D, in which in the
quadratic exponent appears an additional regeneration time
coefficient, the so-called Lea-Catchesid factor *) RG
= 2[(1- e - l .T). (1-1/l.T)] / l.T, which is a function of the rate of cell repair l and irradiation
time T. The rate coefficient of repair l ranges from about
0.4 hours-1
for normal tissues to about 1.5 hours-1 for rapidly dividing tissues (e.g. tumor).
*) Lea-Catcheside dose-time integral :
In the general case, continuous irradiation of a
set of N(t) cells with a time-varying dose D(t) with an
instantaneous dose rate R(t) = DD(t)/Dt can be considered. Then,
for each elementary time interval Dt during which the cells
receive a dose DD = R (t).Dt and N(t). b. DD 2 cells is damaged (sublethal), at the same time it is
enough to regenerate N (t). l. Dt of cells, where parameter l is the rate of cell
repair. By integrating the relevant differential equation from t
= 0 to the continuous time t , under boundary conditions
at t = 0 N(0) = N0, we obtain a solution for the time dependence of the
number of cells N(t), which can also be written in the original
(quadratically) exponential form N(t) = N0 .e - RG(t). b
.D (t)2, where the modifying quantity
RG(t,l) = [2/D(t)2]. 0ntR(t).dt
. 0nt'R(t').e-l.(t-t')dt'
is the so-called generalized Lea-Catchesid function (simplified function of this kind in the years 1942 to
1945 have found empirically D.E.Lea and D.G.Catcheside
when examining the cell aberrations on Drosophile ), expressing the "balance" between cell damage
by dose rate R(t) and their continuous repair with a rate
coefficient of l. The RG function depends on the time course of
the dose rate R(t) during the entire exposure. In extreme cases
of short exposure time T << 1/l is RG ® 1 (repair does
not apply during irradiation), in the opposite case of long
irradiation time T >> 1/l with low dose rate is RG ® 0 (almost all
potentially lethal damage can be repaired).
b -process, in
co-production with cell repair, thus causes the so-called dose
rate effect: that the biological effect of ionizing
radiation depends not only on the total absorbed dose, but also
on the dose rate *), as is schematically shown
in the lower part of Fig.5.2.3c. For low dose rate
(LDR), the number of reparations is high and the curve of the
surviving fraction of cells is relatively flat - less
biological effect (blue curve). The higher the dose
rate, the more likely it is that a break in the second strand of
DNA will occur before the first strand is repaired, usually
causing lethal damage to the cells. For high dose rate
(HDR), therefore, the curve of the surviving fraction is steeper
- a larger biological effect, significantly
increasing with dose (red curve in Fig.5.2.3c below).
*) In the exponential law for b -process N = No .e - RG.
b .D2 the dose rate is output explicitly,
when a quadrate dose D2 = D.D a single dose quantity D we transfer to
Lea-Catcheside factor RG: RG .D = 2.
[(1-e - l .T .) (1-1/l.T)]. D /L.T and realize that the D/T is the dose rate R
. In the general Lea-Catchesid integral, the dose rate R
appears directly.
2. Repopulation
In addition to the exponential decrease in the number of cells
due to radiation damage, there is a continuous replacement of
extinct cells by dividing the surviving cells. Over the time
interval Dt, the number N of existing cells increases by N.n.Dt, where n is the rate
of cell repopulation; the doubling time
T2r = ln2 /n of the number of cells by repopulation is often used.
Integration yields an exponential law of cell number growth by
repopulating N = N0 .e n .T. In logarithmic
form, this leads to another additive term RP = ln2. T/T2r ,
expressing the ratio of irradiation time T and time T2r
doubling of cell number by repopulation. Cell repopulation
further enhances the above dose rate effect.
Including these time effects, the linear-quadratic
dependence takes the final form (on a semi-logarithmic scale) :
- ln (N / N0 ) = a .D + { 2. [(1-e - l .T ). (1-1 / l .T)] / l .T } . b .D2 - ln2.T / T2r .
These relationships between the biological effect and
the radiation dose, including its time course (irradiation time T),
play an important role, especially in radiotherapy,
where fractionation of irradiation doses is used in
order to optimize the resulting radiation response of tumor
tissue with respect to healthy tissue - see §3.6 "Radiotherapy".
An interesting consequence of the above dependences of the LQ
model may be the absence of a deterministic effect
even at relatively high total radiation doses (significantly
higher than the thresholds for single irradiation according to
Fig.5.2.3b), if irradiation is long-term with dose rate lower
than cell repair and repopulation. In this case, only stochastic
effects can occur.
Deviations from the LQ model. Alternative
models and dependencies.
Althout the linear-quadratic model is very well supported by
theoretical analyzes and number of experimental results, it is a
somewhat simplified mechanistic description of complex
processes taking place during ionizing irradiation of tissues and
cell populations. In practice, from the standard (idealized) LQ
model some deviations occurs.
Higher exponents of
the dose
A double DNA break is usually
considered unrepairable, leading to inactivation and mitotic cell
death. However, later experiments have shown that even such
damage can sometimes be repaired by the cell, albeit with less
probability. In addition to the first and second powers, it is
sometimes appropriate to include smaller corrections containing higher
powers (exponents) of the dose in the dose
dependence of the radiobiological effect (Fig.5.2.4a). They could
partly have their origins in multiple interactions
(simultaneous or rapidly consecutive) of quantum ionizing
radiation, resp. their chemical radicals, with cell nuclei that
are more difficult to repair. In reality, however, many different
influences apply here. This is especially evident in densely
ionizing radiation (with a high value of linear LET energy
transfer), where, in addition, the transmitted energy is
distributed unevenly (especially in the region of the
Bragg maximum for radiation a, protons and other heavy charged particles). Dependence
[ln (N/N0)](D)
can then develop into a Taylor series by the various
powers of the dose D .
Tissue
biological influences
Even more significant are some individual biological
influences. Each tissue is in fact a heterogeneous
cell population, containing cells at different stages of the
cell cycle and cells with different radiosensitivity
- with different coefficients a,
b: the resulting survival
curve [ln (N/N0)](D) is then a superposition of several different LQ
curves. In addition, during the actual exposure, so-called cell redistribution
may occur (change in the relative proportion of cells with
different radiosensitivity: a decrease in the
proportion of G1 and G2 cells and total clonogenic cells, while M
and S cells and effector daughter cells will have a relatively
higher proportion) during irradiation, or reoxygenation
(change in oxygen content - the above-mentioned "oxygen
effect"). The oxygen effect is significant especially when
using sparsely ionizing radiation (photon radiation g or X is most often
used), where the indirect radicals mechanism of the radiation
effect predominates. In densely ionizing radiation, where there
is an increased proportion of the direct intervention mechanism
(and also increased radicals recombination), the effect of oxygen
(oxygenation) on the radiobiological effects is less significant.
These biological processes can result in changes in the
radiation sensitivity of cells and tissues during
exposure, leading to further deviations from the LQ model
dependencies.
Bystander effect
The so-called bystander effect of
remote induction of radiation effects in tissue is also discussed
(described above in the section "Effects of radiation at the tissue level"), which may slightly increase the number
of damaged cells (Fig.5.2.4b). The influence of
the bystander effect can be included in the LQ model by inserting
a new empirical coefficient B> 1, indicating the average
number of damaged cells when one cell is hit: N/N0 = e - B ( a
.D + b .D2). However, this is equivalent to a standard LQ model
with slightly higher values of the parameters a' = B.a, b' = B.b. Due to the
bystander effect, the resulting radiosensitivity coefficients a', b´ for the tissue
are therefore slightly higher than the coefficients a, b for individual
cells. Factor B is different for different tissues. In
practical radiobiological experiments, however, radiosensitivity
is determined for tissue (or tissue cultures), where the
influence of the bystander effect is already implicitly
contained: a®a´, b®b´. The LQ model for practical use therefore remains unchanged.
The bystander effect does not change the basic principles and
dependencies of the LQ model, it only causes differences in
radiosensitivity between the cell and tissue levels.

Fig.5.2.4. Some deviations of the dependence of the biological
effect on the dose from the LQ model.
a) Multiple radiation interaction with the cells
resulted in the presence of members of the higher power of the
dose D . b) The bystander effect slightly
increases the total number of damaged cells (decreases the
fraction of surviving cells). c) In the area of
low doses, a relatively increased cell sensitivity -
hyperradiosensitivity - is observed on the graph of the surviving
fraction of cells.
Hyper-radiosensitivity
to low doses. Induced repair model
The higher the absorbed dose of ionizing radiation, the greater
the amount of free radicals generated - the greater the damage to
cells in the tissue or cell population. And the higher the
percentage of cells killed: the dose dependence
of the N/N0
of the surviving fraction of cells should be monotonically
decreasing. However, some radiobiological experiments have
shown that in the area of very low doses (approx. 0.1-0.5 Gy),
the otherwise gradually decreasing, almost linear dependence of
N/N0 of
the surviving fraction of cells (according to Fig.5.2.3c) has a
significant "valley", showing a relatively increased
sensitivity of cells than would correspond to these low
doses (Fig.5.2.4c) - the so-called hyper-radiosensitivity
to low doses for deterministic effects. The cells start to
repair only after a certain dose ...
The detailed mechanism of this
paradoxical behavior is not yet known. It may be related to the
kinetics of enzymes in the G2 phase of the cell cycle during
reparations by homologous recombination (see
the "Nitrocellular Repairs" section above), where the concentration of enzymes increases with
increasing dose, inducing an increased rate of reparations. Thus,
at the cellular level, it appears that cells may need some low
level of damage to initiate the production of repair enzymes by
feedback processes. If at low doses this level is not reached,
the reparations would not work and in the check-point would be
next mitosis stopped ® inactivation of cell ® higher sensitivity to low doses. At a higher dose (and
thus greater DNA damage), the production of repair enzymes would
already take place ® more efficient repair ® relatively lower
sensitivity - the dependence returns to the standard curve of the
LQ model. This phenomenon is sometimes referred to as induced
repair.
Note
1: Some
"physiological defense mechanism" against the mutagenic
effects of radiation in the area of increased probability of
survival of cells with partially impaired genetic information is
also being considered vaguely; these cells die due to
hypersensitivity (in the spirit of the above-mentioned motto for
damaged cells "dead cell = good cell") and do
not endanger the organism with later stochastic effects.
Note 2:
The mechanism of radiation-induced repair could contribute to radiation
hormesis discussed below in the section "Low doses of radiation: - are they harmful
or beneficial?".
Note 3:
A kind of "prototype" low dose hyperradiosenzitivity
perhaps could be called Petkau effect (
phenomenon), who in 1972 described the Canadian
radiobiologist A.Petkau. It was allegedly an increased
destruction of cell membranes during long-term irradiation with
very low doses of radiation. He explained the mechanism of action
of negative oxygen ions, which at lower concentrations damage the
membranes more, while at higher concentrations they have greater
recombination and less effect. This explanation is not very
convincing and the Patkau phenomenon itself was not
confirmed by later radiobiological studies (it was
observed at a time when technical means and radiobiological
knowledge did not yet allow to reliably demonstrate such a
phenomenon or reveal its mechanism; it could be a chemical effect
of carriers of used radionuclide 22Na, or similar side effects). In light of current
radiobiological findings, the primary effect of a low dose should
be on DNA whose uncorrected damage induces cell apoptosis with
secondary cell membrane damage (as discussed in the "Mechanisms
of Cell Death" section above). Not
much was known about cell apoptosis at that time.
Within the LQ model with standard
parameters a, b hyperradiosensitivity can be included by inserting an
initial component a with a higher parameter value ahyper > a - assuming a dose dependence of the parameter a: a(D) = a + (ahyper - a) .e - D/Dhyper, where ahyper is the initial
higher value describing slope [ln (N/N0)](D) for low doses, a
the default value for the higher dose, Dhyper (» 0.2 Gy) is a
boundary area hyperradiosenzitivity expression. The LQ model N/N0 = e - [a(D)
.D + b
.D2] with such a dose-dependent parameter a(D) then captures
the experimental data well. For high doses D >> Dhyper turns into a
standard LQ model with the usual parameters a, b, for low doses D
<< Dhyper behaves as an LQ model with parameters ahyper , b. It is thus a combination
of two LQ models with different a -sensitivities (two
different directives in Fig.5.2.4c), combined into one equation;
it is sometimes called the IndRep model
(induced repair).
Notice: Please do not confuse this
physiological (relative) low-dose
hyper-radiosensitivity, inherent in all cells, with pathologically
increased overall radiosensitivity due to genetic disorders of chromosomal
instability - mentioned above in the section "Dose-biological
relationship".
(multi) Target model
This simple and largely phenomenological model is the predecessor
of the LQ model. It is based on the assumption that there are one
or more radiation- sensitive targets in the
cell. If one of these targets is hit (by radiation or radicals),
it leads to cell inactivation and death. If one such a
target is present, then by an analogous application of Poison's
statistical distribution of the probabilities of intervention,
which we used above for the a - process in the LQ model, we get the dependence of the
surviving fraction of cells N/N0 on the dose D: N/N0 = e - D/Do, where Do is the dose that
leads on average to one hit of the target. If n targets
are present, then the multi-target model of
single-intervention inactivation gives the dependence: N/N0 = 1 - (1 - e-D/Do) n. To
obtain better agreement with experimental values in the low dose
range, sometimes the n-target dependence is combined with the
1-target dependence (each with a different value of the empirical
constant Do).
Kinetic models of DNA
breaks - LPL and RMR model
Models based on the analysis of the kinetics of such DNA breaks,
which may (but may not) result in the killing of cells, come from
the 1980s. Models lethal and potentially lethal effect
(LPL, S.B.Curtis 1989), and corrected/uncorected damage
(repair/misrepair model - RMR, C.A.Tobias 1985), sometimes unite
into so-called model of two lesions TLK (two-lesion
kinetic) are for the usual doses (units up to tens of Gy)
practically identical to the LQ model.
The local effect model
Local Effect Model (LEM ) takes into account the local dose
distribution along the path of ionizing radiation, in relation to
the dimensions and density of the distribution of cell nuclei in
the irradiated tissue. It is based on a simple general
relationship N/N0 = e - N(D), where N(D) is the average number of
lethal lesions per cell at dose D (only the a-process is taken
into account). It then introduces the local volume density of
lethal lesions s(D) = N(D)/V, (where V is
the average volume of cell nuclei), which
relates to the local density of the dose distribution along the
path of the particles for different types of radiation. Through
this analysis model better describes the biological effects of
densely ionizing radiation (proton, a particles, radiation of
heavy ions).
Two-stage stochastic
model
This further improvement in the modeling of the radiobiological
effect was developed by Czechoslovak experts (P.Kundrát,
M.Lokajíèek, H.Hromèíková). As in the classical LQ model, it
is based on the analysis of cell damage by lethal a- process
and less severe damage by b-
process, which is in
principle repairable, but a combination of two b-damage is lethal.
Using Poisson statistics, the probability of the cell (cell
nucleus) hitting a certain number of particles depending on the
dose, LET and size of the nucleus is analyzed, as well as the
probability of cell survival after repair processes. In this
analysis, it is assumed that the condition for the survival of
the cell after a given number of interventions by the ionizing
particle is no damage by the a-
process and a maximum of one damage by the b- process, as well
as its successful repair. The model is elaborated especially for
proton and ion radiation.
High-dose
modifications of the LQ model - LQL, gLQ, USC, KN, PLQ
When irradiated with high doses of radiation
(d > 5, 10 or more Gy/fraction), which allow
advanced conformal techniques of stereotactic radiotherapy and
HDR brachytherapy (§3.5, section "Stereotactic
radiotherapy SBRT"), showed some
discrepancy between expected and observed effects:
the classical LQ model in these at higher doses per fraction
somewhat overestimates the biological effect of radiotherapy,
predicting higher damage to normal tissue NTCP. As if the curves
of the surviving fraction of cells -ln (N/No) (on a log-linear scale) at higher doses actually
showed an increased proportion of the linear component than the
quadratic. To capture this clinical knowledge, some empirical modifications
of the LQ model are sometimes used that better capture
the radiobiological effects in at higher doses :
× LQL
model (linear-quadratic-linear) for higher
doses/fraction (higher than 2.a/b, in practice >approx. 6Gy) adds an additional linear
component increasing the surviving fraction of
cells.
× Generalized gLQ model (generalized
LQ). The standard (conventional) LQ model assumes that the
velocity l repair of radiation-induced sublethal damage
remains constant over time, leading to an exponential repair
pattern in the Lea-Cathesid term RG. However, some experimental
studies suggest that the repair rate of sublethal lesions slows
over time. The generalization in the gLQ model consists in
introducing a reparation term with a reciprocal time of 1/T into
the Lea-Cathesid term RG.
× Universal
Survival Curve (USC), which is compiled for
the high dose area based on a combination of the
LQ model (which gives a good description for small and
medium doses) and the multi-target MT model (better
describing the effect at higher doses - dependence in this area
it is based more as a straight line).
× The KN ( Kavahagh-Newman
) model introduces another dose-dependent factor
into the b- member in the LQ model: ln(N/N0) = -a.D - b.D2.[1 + (b/g).D]-1 .
× PLQ
(Pade Linear Quadratic) model
introduces an additional dose-dependent factor into the overall
expression for the probability of cell survival in the LQ model:
ln(N/N0) =
(-a.D -
b.D2).(1 + g.D)-1
(the name comes from the fact that the expression can be modeled
as a Padé-approximation of the ratio of two
polynomials).
Phenomenological
character of the LQ model ?
All the above-mentioned complex
processes at the level of cells, cell populations and tissues
(whose mechanisms are often not yet examined in detail) cause in
practice, that the coefficients a, b in the LQ model
sometimes lose their unambiguous physical or radiobiological
significance and take on the character of empirical constants.
In the area of radiation doses used in biological applications
(units up to tens of Gy), they good model the real
dependence of the N/N0 of the surviving fraction of cells on the dose, but the
LQ model itself becomes rather summary phenomenological
description of the biological effect of radiation.
Nevertheless, this model, based on a precise analysis of the
relevant mechanisms, remains the best
and most sophisticated model of
dose dependence of the radiobiological effect, even for practical
use..!..
Small doses of radiation: - are
they harmful, or can they be beneficial ?
or
Will the basic
paradigm of radiation protection change ?
" Any irradiation of healthy living
tissue with ionizing radiation, even at a very small dose, can be
potentially dangerous to the body due to its late stochastic
effects. Therefore, it is necessary to reduce radiation doses to
a minimum by all available means. "
In the extreme case, even a single quantum of ionizing radiation
that enters the body, can damage the DNA in some cell and
eventually lead to tumor disease ..?!..
This is the basic starting point of the current
radiation protection, "canonized" in all standards and
regulations for working with ionizing radiation. In this section,
we will try to question this basic paradigm of
radiation protection a bit "heretically" - we will
discuss some alternative views on the risks of
low-dose exposure.
On what
factual data is this starting point of radiation protection
based? In addition to experiments on small animals (rats),
criminal abuses of nuclear forces in Hiroshima and Nagasaki, as
well as a number of radiation accidents, have become a source of
data on the biological effects of radiation. During these tragic
events, many people were irradiated with various doses of
radiation, including very high and lethal doses. Radiobiology and
radiation medicine thus gained a fairly good idea of the deterministic
effects of radiation, the course of radiation sickness
and the possibilities of its treatment. Long-term follow-up of a
number of people, who received lower doses than corresponded to
acute radiation sickness, also provided important information
about stochastic effects of radiation and their
dose-dependence. Further data on deterministic and stochastic
effects of radiation were obtained by monitoring large groups of
patients irradiated in medical therapeutic and diagnostic
applications (radiotoxicity, secondary post-radiation
malignancies).
"Linear
threshold-free theory" is only an unsubstantiated hypothesis
A common feature of most studies on the biological effects of
ionizing radiation is that these are medium, higher,
and high doses (from a few tenths of Sv to many
tens of Sv). For these values it is possible to construct a
fairly objective relationship between the dose value and
biological effects (Fig.5.2.3). As for the lowest doses
(at the level of units and tens of mSv), it must be admitted that
it is a "terra incognita", where reliable data
are lacking *); it is not surprising, because the label
"stochastic effects" itself says, that it is difficult
to find any causal regularities here ...
*) For medium and higher doses (in the area
above about 400mGy) the relationship between dose and effect is proven
and statistically significant. However, towards lower doses, the
results are blurred due to statistical fluctuations and
uncertainties; for the lowest doses, the biological effect is
no longer statistically detectable. Given the assumed
roughly linear dose-effect relationship, the number of
individuals required to demonstrate the statistical significance
of a stochastic effect increases with the inverted square of the
average dose required to produce the effect. If the effect at
doses around 1 Gy is demonstrable in a statistical group of
several thousand people, then the demonstration of the effect
conditioned by a dose of 0.1 Gy requires a hundred thousand
group. For the lowest doses close to zero, the existence of a natural
radiation background completely makes it
impossible to analyze the relationship between dose and
radiation effect. Furthermore, a number of disturbing side "masking"
factors can manifest themselves here, completely
distorting the observed results!
Conservative
radiobiologists and radiation protection experts will therefore
take the point [0,0] as a starting point (zero dose = zero
effect), interpolate the linear dependence from zero to the
actually proven values and replace the missing values
in the lowest dose range with extrapolated hypothetical
values (straight line on Fig.5.2.3 left). And right here
arise a claim about the harmful stochastic effects even the
lowest doses of radiation, or the thresholdlessness
of stochastic effects. Statement often repeated (and therefore
generally accepted), but unproven ! Linear
threshold-free theory is not a radiobiological
hypothesis, but is the result of processing and straightforward
approximation of available data.
Careful
analysis of the data collected on dose-response relationships
shows that the dose-response is probably not linear.
The effects in the area of low doses are probably smaller
than would correspond to the previously assumed linear dependence
(a blue or green curve in Fig.5.2.3 on the left is possible
closer to reality). The assumption of linear dependence and
thresholdlessness of stochastic effects, on which the current
concept of radiation protection is built, represents a conservative
approach, overestimating the risks in the area of small
doses.
Let's turn
around to an argument from another direction - the development
of life on Earth and the role of repair processes in
cell nuclei. The basic mechanism of genetic transmission of
information in primitive cells evolved hundreds of millions of
years ago, when the level of natural ionizing radiation
was much higher than today (higher natural
radioactivity, lower shielding ability of the atmosphere against
cosmic radiation). This high level of radiation background was
both an important driving force in the evolution of life
(mutations + natural selection ® development of new
species), and forced cells to develop sufficiently effective
repair mechanisms against radiation damage. And they
probably retained these mechanisms its
effectiveness to this day. Even now, due to natural radioactivity
and cosmic radiation, our organism is irradiated with several
thousand quanta of ionizing radiation every second.
A large
amount of reactive radicals and oxidants are formed as products
of normal metabolism in the body; there are even
many more than when irradiated with low or medium doses of
ionizing radiation (it is estimated at hundreds of thousands of
DNA nucleotide damage in each cell per day). It can therefore be
expected that harmful radicals caused by a small dose of ionizing
radiation will be lost "like a drop in the
sea" of a large amount of harmful products of normal
metabolism.
Note: This
roughly applies to "sparsely" ionizing radiation.
"Densely" ionizing radiation causes double DNA breaks
that are less repairable, leading to higher radiobiological
efficacy, including a higher risk of stochastic effects.
Radiation
hormesis and adaptive response
In addition, some radiologists believe that a reasonable number
of disorders caused by a small dose of radiation (especially
sparse ionizing radiation) can initiate and stimulate
chromosome-level repair mechanisms in the
organism, which repair not only these radiation disorders, but
can also repair even many other defects caused by metabolim, that
might otherwise remain uncorrected. This radiation
hormesis *), or adaptive response or radiation-induced
repair at low irradiation of cells and organisms, is a
kind of "immunization" in the organism.
*) Hormesis
The word hormesis comes from Greek "hormaein"
= "arouse, excite, strengthen". This is an
experience-based phenomenon in which low doses of some toxic
substances not only do not damage the organism, but even improve
its physiological functions. Hormesis probably arose during
evolution as one of the ability of organisms to respond to
adverse environmental conditions - cold, heat, lack of food,
toxic substances -- factors that disrupt the functioning of the
organism. For this stress factors the organisms often respond by adaptation.
Exposure to small amounts of toxic substances, that damage
biological molecules, triggers a stress response and can lead to hormesis;
the organism then better tolerates higher doses of the given
toxic substance. One of these factors stimulating hormesis, could
be also ionizing radiation ...

Fig.5.2.3. Dependence of biological effect
on the size of absorbed radiation dose
a) Probability of occurrence for stochastic
effects. b) Severity of damage for deterministic
effects.
c) Dose dependences of the surviving fraction of N/N0 cells according to a
linear-quadratic model.
This image we have shown here again for clarity; these are mainly
fig.a) on the left - very low doses.
According to these opinions (but also not yet
reliably proven), small doses of radiation could even be beneficial
for the organism! A low dose of radiation triggers the
"signal" emitted by the damaged DNA, and the subsequent
repair process corrects any errors found (and removes those
nucleotides that cannot be repaired), not just those caused by
the radiation. If the amount of damage caused by the radiation is
less than the "capacity" of the repair system, even
other errors will be corrected; after irradiation with a small
dose, there are fewer errors in the DNA than before - there is a positive
effect (the section of the green curve below the horizontal axis
in Fig.5.2.3a). At a higher dose, when the extent of radiation
damage exceeds the "repair capacity", the effect of
irradiation is already negative (the rest of the
green curve in positive values).
Several mechanisms can act in the radiation adaptive response :
¨ Time
- slowing down the cell cycle, so that by
the beginning of the next mitosis, some damages can be repaired;
¨ Chemical - neutralization and thus
detoxification of reactive radicals;
¨ Biochemical -
activation of enzymes involved in intracellular repair - the
above-mentioned radiation-induced repair
;
¨ Immune - stimulation of
defense mechanisms at the level of the whole organism, which
could help eliminate cells changed not only by radiation.
A certain small "preparatory" or
"preventive" dose of radiation can thus protect
the cells against damage caused by a subsequent much
higher dose. A small dose activates enzymes in the cell, designed to repair genetic
information, making it easier for the cell to deal with the
possible consequences of stronger irradiation. Regarding the
effect of radiation on complex multicellular organisms, there is
also the opinion that a small dose of radiation or some toxins
deprives the organism of cells that have weakened repair
mechanisms and which would be in danger of succumbing to
malignancy in the future.
These (so far)
non-conforming opinions were also verified experimentally.
Of course, it would be ethically unacceptable to perform such
experiments on humans, and not even on higher animals. However,
interesting experiments were performed on bacteria.
The bacterial culture was divided into two parts, one of which
was placed in a well-shielded box, where the level of radiation
background was lower than in nature. The second group of bacteria
was exposed to a weak field of ionizing radiation under the same
other conditions (temperature, pressure, humidity, nutrients). A
surprising result was found: bacteria exposed to
radiation prospered somewhat better than bacteria from the second
group shielded from radiation! However, it was a question of
monitoring the entire culture of bacteria (the reproduction of
which could show selective effects), not of monitoring individual
effects on individual bacteria.
It is clear
that these experimental results and speculative considerations
cannot be straightforward transferred to the biological effects
of radiation for humans. Bacteria are prokaryotic organisms
which, due to their unicellularity, cannot, of course, develop
tumors. We are eukaryotes with a large number of cells of
different species, interconnected by a number of complex
signaling pathways; it is this circumstance that enables the
development of tumors due to cytogenetic mutations, including the
action of ionizing radiation. However, much here may depend on
the function and extent of radiation-induced repair.
Only further
research in the field of molecular biochemistry and cytology, as
well as more extensive epidemiological studies, will hopefully
help to clarify the question of the effect of small doses of
radiation. Until this is reliably clarified, it is in view reliability
of radiation protection, it is necessary to apply in
practice a linear threshold-free theory, which in the area of
small doses is associated with a slight overestimation of risk
and a low probability of possible underestimation.
However, if
the above conclusions are demonstrated in further experiments and
subsequently in clinical studies, a change in the
conservative paradigm of current radiation protection in
the field of low radiation doses can be expected in the future
and subsequently a revision of relevant
standards and regulations. However, radiophobia,
which is deeply rooted in Western society due to the mass media,
will probably persists for a very long time...
Time
course and types of biological effects of radiation
In terms of the time of boarding and time course of the effects
of radiation on the organism, or its tissues and organs, we
distinguish two groups - early and late :
× Early effects of
irradiation
develop within a relatively short time (days to weeks) after a
one-time irradiation with a larger dose of radiation. They are
caused by the extinction of a significant part of the cells of
irradiated tissue, especially stem cells, in rapidly
proliferating tissues, in which there is a need for continuous
rapid renewal of daughter effector cells that have a short life
cycle. Stem cell depletion soon results in a
loss of effector cells, which is reflected in deterioration of
irradiated tissue function. These tissues (eg hematopoietic
tissue or mucosal cells) have a high radiosensitivity and a rapid
response to irradiation. These are acute deterministic effects
and the rule is that the higher the dose, the earlier
the effects start and the more severe they are.
If too many stem cells are not damaged, after overcoming these
early effects, there is also a relatively rapid recovery
of the tissue by cell repopulation (accelerated stem cell division, or a temporary loss of
division asymmetry in favor of effector cells) and its return to normal function. However, late
radiation effects (deterministic and stochastic), discussed
below, may occur over a longer period of time.
Very early radiotoxic
effects
When exposed to a high dose of radiation all over the body, very
early symptoms of the body's stress response, such as fatigue,
nausea, and dry mouth, may appear very quickly, within a few
hours. These manifestations of very early radiotoxicity
they are not caused by the mechanisms of radiation killing of
cells - although a larger number of cells are damaged, but this
damage will appear later, only during the mitosis of these cells.
Very early radiotoxicity is caused by irritation of the
body's regulatory centers (nervous and humoral) by
direct exposure to released ions, radicals and other
products of radiolysis. At very high doses
(hundreds of Gy one-time), very rapid and fatal radiotoxic
effects can occur due to denaturation of the cell contents
(rapid necrotic cell death) and the intercellular environment.
The
early effects of radiation - early radiotoxicity
- include :
- Acute radiation
disease
(acute post-radiation syndrome)
Occurs after a one-time irradiation of the whole body (or
most of it) with doses greater than about 1 Gy. The
clinical course here depends on the dose. At doses from
about 3 to 8 Gy, blood form is formed
(marrow, hematological) caused by damage to hematopoietic
organs. The number of white blood cells and lymphocytes
in the blood picture decreases significantly, as does the
number of red blood cells and platelets. If islands of
functional hematopoietic stem cells are preserved in the
bone marrow, their division can lead to gradual
regeneration after 6-8 weeks. At doses around 10Gy,
hematopoietic cells are usually irreversibly destroyed,
the dose is lethal, if the bone marrow cannot be
successfully replaced by transplantation.
In addition, at doses higher than
10 Gy, an intestinal (gastrointestinal)
form of radiation sickness develops, caused by
destruction of intestinal lining cells. Here there are
serious disorders of the body's management of fluids and
minerals, with the possibility of bacterial toxemia.
At even higher doses at the levels of tens
and hundreds of Gy, the neural or neurovascular
form of acute radiation sickness occurs very quickly
after irradiation, associated with the extinction of more
nerve cells (direct nerve cell damage or hypoxia due to
vascular damage), followed by orientation and
coordination disorders, unconsciousness and death. The
cells are already killed by necrosis in the interphase,
which causes acute inflammation.
Acute radiation disease has four
time stages :
- The early stage, also called prodromal,
mentioned above; it fades very quickly.
- Latent phase
during which no subjective difficulties occur. As the
radiation dose increases, the latent phase shortens.
- A manifestation phase in which
the full development of radiation sickness, caused by the
death of a large number of cells, takes place. There is
increased fatigue, temperature, bleeding, inflammation of
the mucous membranes, the possibility of developing
bacterial and viral infections. This phase lasts about
4-6 weeks.
- Reconvalescence phase
in the favorable case, where, depending on the severity
of the irradiation disease, partial or complete recovery
is gradually taking place. Killed cells are replaced by
dividing healthy cells.
- Acute radiation
dermatitis
When irradiating the skin with doses above 3Gy, 1st
degree dermatitis occurs - erythema (redness) of the skin
and hair loss, at doses above 10Gy then 2nd degree
dermatitis associated with blisters and ulcers on the
skin, further necrosis (dermatitis 3.degrees). Radiation
dermatitis is accompanied by degenerative skin changes
and is difficult to heal.
- Radiation inflammation ,
by which the tissue can respond both to rapid termination
of larger number of cells, with the formation of cellular
decomposition products.
- Impaired fertility
- Embryo and fetal
damage
The human embryo and fetus, formed by intensively
dividing cells, is very sensitive to
ionizing radiation; it can be damaged even when
irradiated with a dose of 0.1 Gy. The nature of damage to
the fetus by ionizing radiation depends, in addition to
the amount of radiation dose, mainly on the stage
of development, ie on the time elapsed since
fertilization.
In the initial stages (in
the first 3 weeks), when the number of cells in the
embryo is still small and their function is not
specialized, their radiation damage usually results in
the death of the embryo and termination
of pregnancy. The nature of this effect in the early
stages of the embryo is sometimes referred to as "all
or nothing" - the embryo either disappears or
survives without sequelae.
During the period of major organogenesis
(weeks 3 to 8), irradiation can cause malformations
in the developing organs of the embryo. When the fetus is
irradiated during the 2nd-6th month of pregnancy, there
is a risk of developmental disorders and
mental disability of the newborn.
In the last trimester of pregnancy,
relatively higher radioresistance occurs. In any case, it
should be noted that from the 4th week, exposure of the
fetus leads to a significantly higher risk
stochastic effects of a tumor nature,
2-3 times higher than that of adults. These tumors may
then manifest in childhood or adulthood.
× Late effects of
irradiation
can manifest themselves after several months, years to decades of
latency from irradiation. They arise either as deterministic
effects after intense irradiation, long-term or repeated
exposure to smaller doses of radiation (non-tumor late damage - late
radiotoxicity), or as stochastic effects
(cancer and genetic disorders). Late deterministic effects are
caused by tissue damage with slow effector cell regeneration and
thus low stem cell proliferative activity. Damage to stem cells
by irradiation will only become apparent when their mitosis is
needed to replenish effector cells, which is usually several
months apart. Only then do damaged stem cells die and the tissue
begins to show a lack of effector cells. Tissues of this type
(such as connective tissue, muscle tissue, kidney) with slow cell
division have low radiosensitivity and slow response to
irradiation. However, the effects persist for a long time,
sometimes even permanently.
Combination of early
and late radiation effects
Individual organs are often made up of different types of
functionally connected tissues - reacting quickly and late,
within one organ. Therefore, early acute radiotoxicity occurs
during irradiation, after its disappearance, subsequent
(consequential) late radiotoxicity may manifest over time. E.g.
at irradiation of the lungs, after the acute radiation
pneumonitis, late lung fibrosis may gradually develop.
The late effects of radiation include the
following types of radiation damage (the
first two are deterministic, the second two are stochastic) :
- Chronic radiation
dermatitis - occurred
especially in radiologists who performed sciascopic X-ray
examinations without sufficient protection.
- Lens opacity (cataract) may develop following exposure of
the eyes with doses higher than about 4-8 Gy.
- Malignant tumors - are the most serious late somatic effects of
stochastic nature. They can arise as a result of mutations
that result in loss of control over cell division and malignant
transformation of affected cells - §3.6 "Radiotherapy", passage "Carcinogenesis".
- Genetic changes can be manifested by the disability of the
offspring of irradiated persons due to mutations in germ
cells.
Local tissue and organ radiation
effects
Biological effects of radiation, we first analyzed at the molecular-cellular
and tissue level in general (see above
"Effects of radiation on cells",
"Radiation effects during the cell cycle"). We have shown different sensitivity of cells in
individual phases of the cell cycle and the resulting difference
in radiosensitivity for stem and effector cells, as well as for
different tissues and organs. We further analyzed the dependence
of biological effects on the size of the absorbed dose and its
time course ("Linear-quadratic
model of deterministic radiation effect"). Then we focused on the radiation effects on the
organism, especially in whole body irradiation.
However, a frequent case of
radiation exposure is the dominant exposure of only a
certain part of the body, a certain organ or tissue.
Especially in the case of radiotherapeutic use
of ionizing radiation (§3.6 "Radiotherapy"), a carefully selected part is exposed - the tumor
lesion (target volume), while the surrounding healthy tissues and
organs also receive certain doses. In these cases, specific
aspects of radiation sensitivity and the response of different
tissue and organ types to different spatio-temporal
dose distributions are very important. From the
point of view of the nature of sensitivity to the spatial
distribution of the radiation dose in tissues and
organs, it is important "organizational structure"
and the size of individual functional parts. In general,
organisms are composed of cells, grouping into tissues and
functional units - organs. Organs
consist of smaller groups of cells that provide function in
individual parts of the organ - the so-called functional
subunits. Functional subunits have different
sizes and are differently interconnected and organized within a
given organ, event. its links to other tissues and organs. According to the arrangement of functional subunits,
biological organs can be divided into two basic types :
¨ A serial
organ
is made up of a number of linearly arranged cellular structures
(functional subunits), which are functionally arranged one behind
the other - in series. The specialized activity
of the cells of one section functionally follows the activity of
the cells of the previous section. Local damage to even a
relatively small place (section) can lead to a "outage"
of the function of the entire subsequent rest of the organ.
Examples of serial organs are spinal cord, optic nerve,
esophagus, ureter, intestine and the like.
¨ A parallel
organ
consists of many cellular structures (functional subunits) of
roughly the same function, which occupy a larger space
and are arranged side by side (in parallel
), working "together". Local damage to a smaller volume
of such tissue is not significantly reflected in the activity of
the whole organ, the remaining cells are enough to functionally
cover its activity for the organism - these organs have a
significant functional reserve. Examples of
parallel organs are the liver, lungs, kidneys, thyroid gland,
spleen, etc.
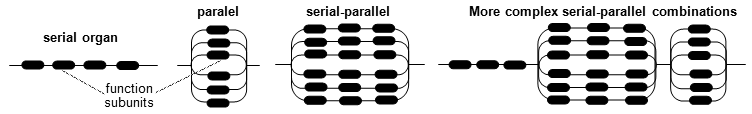
In a detailed view, the series and
parallel arrangement is partially combined - for
example, the parts connected in parallel with the local series
arrangement of the elementary functional subunits. And organ
systems or tracts (GIT, urinary tract) are a complex
series-parallel arrangement of many different parts and
functional subunits.
Serial and parallel organs have different
radiobiological behavior in terms of spatial
distribution of radiation dose. Serial organs may relatively well
tolerate a dose evenly distributed over its entire volume, or
whole body dose, but are very sensitive to high local
dose, which can "exclude from the function"
them. Parallel organs behave in the opposite way, which tolerate
even a very high local dose to a small volume (the elimination of
which will reduce the function only insignificantly), but they
are damaged by the high total dose to their
entire volume, resp. it depends on the total volume of
irradiated tissue. This different nature of the radiation
sensitivity of serial and parallel organs to the spatial
distribution of the dose is of great importance in radiotherapy,
where serial organs in particular often act as so-called critical
organs, for which the tolerance dose
should not be exceeded (see §3.6 "Radiotherapy").
Sources
of irradiation with ionizing radiation
Throughout the duration and evolution of life on Earth, all
organisms are exposed to ionizing radiation from natural
sources - cosmic radiation (§1.5.,
part "Cosmic radiation") and natural radionuclides (§1.4., part "Natural radionuclides"). The same is true
throughout the existence of man. Since the beginning of the 20th
century, after the discovery of X-rays and radioactivity, in
addition to natural sources, is comming also a number of artificial
sources of ionizing radiation, which are increasingly
used in many fields of medicine, research and industry.
Depending on their physical and chemical
properties, sources of ionizing radiation cause either external
or internal irradiation. External irradiation is
caused by sources located outside the body, internal irradiation
is caused by radiation from radionuclides found inside the human
body. The way of the irradiation and specific doses of radiation
further depend on the occurrence and movement of individual
sources (natural and artificial) in the environment.
N
a
t
u
r
a
l
---
A
r
t
i
f
i
c. |
Radiation
source |
Effective
dose
[mSv / year] |
share
[%] |
88%
-----
12%
|
| Radon (and its decay products) |
2.1 |
48 |
| Terrestrial radiation |
0.45 |
17 |
| Internal irradiation with natural
radionuclides in the body |
0.25 |
9 |
| Cosmic radiation (secondary) |
0.4 |
14 |
| -------------------------------------------- |
------------- |
---- |
| Medical irradiation (diagnostics,
therapy) |
0.3 |
11 |
| Occupational exposure |
0.002 |
0.08 |
| Technical and consumer items |
0.005 |
0.02 |
| Nuclear energy (excluding accidents) |
0.001 |
0.04 |
| Radioactive fallout (nuclear weapons
and accidents) |
0.005 |
0.02 |
| Approximate values of the average
radiation dose from individual radiation sources related
to 1 person per 1 year |
The average annual dose from irradiation from
natural sources for humans is about 3.2 mSv, from artificial
sources it is estimated at about 0.3 mSv/year. At present,
therefore, on average, about 90% of the radiation exposure is
from natural sources and 10% from artificial sources. In any
case, overall these are very small doses of radiation.
Natural
sources of ionizing radiation
In our environment, we are constantly exposed to ionizing
radiation from cosmic radiation and from natural
radionuclides in the air, in surrounding objects, in soil and
rocks, building materials and in our bodies. Natural sources
causing radiation dose in living organisms and humans can be
divided into three components according to their nature and
significance :
- 1. Internal
irradiation by natural radioisotopes
In each organism there is a certain very small amount of
natural radionuclides, which by their ionizing radiation
cause internal irradiation. Terrestrial
and cosmogenic radionuclides enter the body through the food
we eat, the water we drink and the air
we breathe *). Chemicals containing radionuclides then
enter the metabolism, some are again excreted
rapidly by respiration and excretion (especially in the urinary tract), others are excreted more slowly and may accumulate
in certain tissues (especially bones). These are mainly
radon and potassium 40K.
*) All nature (terrestrial and
space) contains a small amount of radioactivity,
especially natural radioactive elements. Compounds of
primary and secondary radionuclides (radioactive decay
series) enter the soil, water
and air
(especially through gaseous radon) from
rocks during their erosion. During the combustion
of coal, some natural radionuclides are released into the air (especially radon 222Rn, polonium 210Po and lead 210Pb), which accumulate at
greater depths due to the decay of the primary natural
radionuclides uranium and thorium. During volcanic
activity, the ejected clouds of volcanic smoke
and dust contain a significant amount of radon
radionuclides, polonium-210, lead-210 from great
underground depths. Compounds of cosmogenic
radionuclides also enter the biosphere from the
atmosphere. From soil, water and air, natural
radionuclides get into plants and in the food chain into
animals and humans.
Radon and its decay
products
Radon 222Rn is a radioactive gas that is formed by the
decay of radium 226Ra (this radium is one of
the products of the uranium decay series). 222Rn is a -radiator with a half-life of 2.8 days and
decays at a number of other radioactive elements uranium
decay series. Radon itself, as an inert gas, is largely
exhaled again after inhalation, but radon daughter
products (such as 218Po) are absorbed and trapped in the air on dust
particles - when inhaled they settle in the lungs
and bronchi and irradiate these tissues for a long time
with radiation a of high energy (approx. 7MeV) and radiation
efficiency.
Trees - radon
pumps ?
Radon is partially soluble in water, so it passes from
rocks to groundwater. With their roots, the trees absorb
this groundwater with dissolved radon and evaporate it
into the atmosphere through leaves or needles. Trees,
especially those with a large and deep root system, can
act as "radon pumps", that
pull radon out of the ground and release it into the air.
It is estimated that in this way the trees are
responsible for about 20-30% of airborne radon,
especially in densely forested areas. In the wood of the
trees themselves, isotopes of uranium decay series almost
do not accumulate, but there is potassium 40-K and mainly
14-C.
In the open
atmosphere, radon disperses rapidly, but in enclosed
rooms and spaces it can concentrate
significantly. The main sources of gaseous radon are
cavities in the ground (subsoil), minerals (containing
uranium and thus radium) and building materials created
from these minerals. The risk of radon is geographically
very variable (an increased "radon
index" occurs in regions with uranium ore
deposits).
Amount of radon in living rooms (if there is an increased occurrence of radon in
the area, or in the building material used) can be reduced by some technical modifications
- sealing cracks in floors and basements, insulating
masonry coatings, but especially by ventilating
the spaces under the floors and in the lowest
places (radon is a heavy gas, it accumulates below). The
average annual effective dose of radiation from radon is
estimated at about 2.1 mSv, but it is very variable
depending on the location.
Other natural radionuclides inside the body
Another internal radionuclide is potassium 40K, which, together with common
inactive potassium, is contained in the tissues of the
body (the adult human body contains about 150 g of
potassium, of which about 0.012% 40K with a total activity of about 4kBq).
Furthermore, they are, to a lesser extent, cosmogenic
radionuclides carbon 14C and tritium 3H, as well as radionuclides of uranium decay
series 238U and thorium 232Th - 226Ra, 222Rn, 210Po, 210Pb and others; however, radon has been discussed
above in a separate paragraph. The total dose of these
internal radionuclides (excluding radon) is estimated at
0.2 mSv/year.
- 2. Terrestrial
radiation
Natural
radionuclides are present in the soil, rocks,
water and atmosphere, which by their radiation g (and
partly also by radiation b, if it has a high
energy) contribute to the external
irradiation of the organism. These are mainly the primary
radionuclides thorium 232Th and uranium 238+235U with their decay products, and the already
mentioned potassium isotope 40K. The average dose from this external
terrestrial radiation for humans is estimated at about
0.45 mSv/year.
- 3. Cosmic
radiation
Cosmic radiation is an
important and permanent component of the natural
radiation background. Primary and secondary cosmic rays,
including the production of cosmogenic radionuclides, are
described in more detail in §1.6 "Ionizing
radiation", section "Cosmic rays". The
average dose from secondary cosmic rays to humans is
estimated at 0.4 mSv/year. Individually, however, the
dose rate significantly depends mainly on the place in
the atmosphere, ie on the altitude. At ground
level (0 m): 0.03 mSv/hour, at a height of 2 km: 0.1 mSv/hour, at
an altitude of 6 km: 1 mSv/hour, at a height
of 10km: 5 mSv/hr., in 15km: 10 mSv/hr. Secondary
cosmic radiation poses a significant radiation exposure
for aircraft personnel moving at altitudes of around 10
km; primary cosmic radiation burden the spacecraft crews.
- 4. Atmospheric
discharges
Minor amounts of X and gamma radiation occur during
atmospheric discharges - lightning
between storm clouds and the ground (see §1.6., section
"Sources of ionizing radiation", passage "Electric discharges in
the atmosphere"). These are short flashes of g radiation,
which due to their sporadic occurrence do not
significantly contribute to the radiation load compared
to the continuous radiation background - radon, cosmic
and terrestrial radiation (even for an aircraft that gets
into storm clouds, there are other far more serious
risks...).
Artificial
sources of ionizing radiation
Ionizing radiation from artificial sources can be divided into
three categories according to its nature and radiation
significance :
- Medical
applications of radiation
The use of ionizing radiation for diagnostic and
therapeutic purposes in medicine
is the most important of all artificial radiation
sources. These are mainly X-rays in X-ray
diagnostics and therapy, radiotherapy
with g- radiation and diagnostic and therapeutic use of
open radionuclides in nuclear medicine.
All these methods were described in detail in the
individual chapters of our monograph "Nuclear
Physics and Physics of Ionizing Radiation". The
total average dose from medical irradiation is estimated
at about 0.3 mSv/year (however, it is very strongly
individually and geographically dependent). The doses for
individual methods of diagnostic use of radiation in
radiology and nuclear medicine are given in more detail
below in Table 5.7.1 in §5.7 "Radiation exposure during
radiation examinations and therapy".
- Technical and
consumer objects
Products of modern technology, which we increasingly
encounter in everyday life, can emit a small amount of
ionizing radiation. This radiation is generated there
either electronically (television,
computer, instrument screens - this only applies to
vacuum CRT screens), or they may
contain small amount of radioactive substances in closed
or open form (eg fire detectors, radioactive luminaires).
.......
- Nuclear technology
Nuclear reactors, using uranium fission reactions mainly
for energy production (see §1.3. "Nuclear
reactions", section "Fission of atomic nuclei"), increase the radiation level only slightly
during normal operation in the surrounding environment by
leaving air and water only a very small amount of
radioisotopes. The impact of nuclear power plants on the
environment is constantly checked by monitoring of
runoffs and the entire vicinity of the nuclear power
plant.
Radioactive
waste from reactors
One of the main problems of current nuclear energy is spent
nuclear fuel, which contains a high number of
radioisotopes, often with a very long half-life. Their
escape into the biosphere is a potential risk for a long
time. In addition to relatively short-lived radionuclides
(such as 131I with T1/2 8 days), there is a large amount of eg 137Cs (T1/2 30 years), 90Sr (T1/2 28.8
years), 241Am (T1/2 458years), 239Pu (T1/2 2.104 years), 240Pu (T1/2 6.103 years) and a number of other long-lived
radionuclides. In principle, these hazardous radioactive
wastes can be managed in two ways :
1. Storage of this waste in a safe
repository, which should ensure that long-lived
radioisotopes contained in spent fuel do not enter the
biosphere for several thousand years. This is by no means
easy to ensure technically - high requirements are placed
on the tightness and resistance of packaging to
corrosion, the storage must be suitable from a geological
point of view.
2. Rework spent nuclear fuel, in which some components of
spent nuclear fuel can be reused and the majority of
long-lived radionuclides can be converted
to other isotopes that are either stable or have
significantly shorter half-lives. Spent nuclear fuel
would thus cease to be a difficult waste, but it could
even become an important raw material. In
this direction, the so-called transmutation
technologies with the use of neutrons produced
by irradiation with powerful accelerators could be
promising , see the relevant passage in §1.3
"Nuclear reactions".
Nuclear accidents
and nuclear weapons
The above-mentioned favorable radiation situation during
the trouble-free operation of nuclear technologies can
change dramatically in the event of a nuclear
reactor accident or the misuse of nuclear energy
in the service of crime and war - testing or even the
application of a nuclear weapon. In such
a case (in addition to other destructive effects), there
will be widespread contamination, in
which large amounts of radioactive substances enter the
air, which gradually reach the earth's surface
(especially with rainfall) as a radioactive
fallout that contaminates soil and water for a
long time.
Above-ground tests of nuclear weapons were carried out in
the 1950s and 1960s, but were stopped in 1963. Severe
radiation accident, associated with extensive
contamination, was the accident at the Chernobyl
nuclear power plant in 1986 (human factor). Newer
reactors are no longer in danger of such an accident. Another
less serious accident occurred at the Fukushima
nuclear power plant in 2011, as a result of a natural
disaster. This events are described below "Radiation accidents").
The average dose of
radiation from radioactive fallout of this origin does
not exceed about 0.3% of the dose from other sources.
- Professional
exposure
Professional exposure of
people to ionizing radiation began almost immediately
after the discovery of X-rays and radioactivity, at a
time when the risks and harmful effects of this radiation
were not yet known, radiation doses were not known or
measured, workers did not protect themselves from
radiation. Already W.C.Röntgen has undoubtedly received
relatively high doses of radiation in his experiments
with cathode ray tubes, even higher doses of Mrs. M.Curie
in the isolation of radium, and many of their followers.
Very high doses of radiation, associated
with deterministic effects and permanent damage
to health, received the first few generations of
radiologists who did not protect themselves. Likewise,
many nuclear physicists in experiments in the 1920s and
1940s; there have even been a number of fatal radiation
accidents. During the 1930s - 1950s, the harmful
biological effects of ionizing radiation were studied and
irrefutably proven, which led to the gradual constitution
of radiation protection procedures and
standards. Adherence to the rules of radiation
protection, together with an increase in the
technological level of radiation equipment, devices and
methods, has led to a significant reduction in
the radiation exposure of workers with ionizing
radiation, often to virtually zero (natural background
level).
Irradiation of workers with
ionizing radiation occurs mainly from artificial sources
- X- ray equipment, the use of radionuclides for
diagnostics and therapy, nuclear reactors, particle
accelerators, etc. However, it can also be irradiated
from natural sources, which are intentionally used - for
example, for workers in uranium mines, ore processing
plants, etc. All these exposures of persons when working
with ionizing radiation are controlled and regulated by
the relevant standards and regulations
of radiation protection (see below), radiation
dose limits are set for workers.
Radiation monitoring is used to verify
and optimize radiation protection of workers, the working
environment and radioactive waste. The average annual
dose of radiation workers from professional irradiation
is now estimated at about 1 mSv.
5.3.
Objectives and methods of radiation protection
Basic goals of
radiation protection
Proven harmful deterministic effects of strong
radiation *), as well as the risk of harmful
stochastic effects of weak radiation (apart
from the above-mentioned alternative possibilities of low
radiation doses), leads to the need for protection
against ionizing radiation. This radiation
protection or radiation hygiene
represents a system of technical and organizational measures to
reduce unwanted exposure of physical organisms (especially
persons) and to protect the environment from this radiation.
*) By this we mean, of course, the harmful effects of radiation
on healthy tissue, not the targeted
deterministic effects on pathological foci in
radiotherapy that are beneficial for the
organism (see chapter 3.6 "Radiotherapy")!
The basic goal
of radiation protection can therefore be formulated as
follows :
| The
aim of radiation protection is to eliminate the
deterministic effects of ionizing radiation and to reduce
the probability of stochastic effects to a reasonably
achievable level . |
The risk from ionizing radiation is additive
to the other risks (complementary), that we are exposed
to during our lives - environmental and food pollutants, smoking,
genetic influences, infectious and other diseases, etc.
From a statistical
point of view, these risks are sometimes compared
against certain nominal risk factors. These
nominal risk factors are determined statistically
from a certain additional frequency of illness or death depending
on the risk factors. However, the accuracy and objectivity of
such determinations is uncertain and debatable,
there are a number of factors and selection effects,
often unknown. Therefore, in our physically
focused treatise on the effects of radiation and radiation
protection, we do not address these issues.
The
specific nature of ionizing irradiation of the organism
Compared to most other pollutants that affect our organisms,
ionizing radiation has its distinctive specifics :
¨ Ionizing
radiation is not detectable by our senses
If a person is in a place exposed to ionizing radiation, he doesn't
feel it at all. And even at high intensities *), when
the radiation dose soon exceeds the lethal value! If we do not
have a suitable radiometer "on hand", we cannot assess
the danger of this situation.
*) At high radiation intensities, ozone,
which can be smelled in the air, can be a warning signal.
¨ Initial symptoms of radiation
sickness
is virtually indistinguishable from the initial symptoms of
common diseases such as the common cold or flu. If radiation is
not suspected, radiation sickness may not be detected in time and
treated adequately.
¨ Prolonged
clinical manifestation of late effects of radiation
at doses up to about 3Gy, and especially stochastic effects, may
lead to the fact, that we will not associate any health problems
with radiation in the past.
¨ Low level of knowledge
in the fields of nuclear physics, radioactivity and ionizing
radiation in the general human population. This leads to an
underestimation or overestimation of the risks of radiation and
radiation protection, the emergence of various misinformations
and false rumors (such as radiophobia and opposition to nuclear
energy).
Principles
of radiation protection
In general, three basic principles are used to ensure the
objectives of radiation hygiene :
- Principle of justification
In activities leading to irradiation with ionizing
radiation, it is necessary to ensure that such exposure
is justified by a benefit that balances
(or better outweighs) the risks that arise from this
radiation activity.
An objective assessment of the justification for
radiation exposure can be quite complex
and debatable. Many factors of "benefits",
"losses" and costs come into play here (it is
also related to the following aspect of optimization),
some of which cannot even be quantified or measured.
Nevertheless, similar "calculations" are
sometimes performed (using a formula in which
"losses" from radiation risk, radiation
protection costs and possibly other items, are subtracted
from the positive result of radiation activity); but a
similar analysis is subjective in the
real situation, they often compared "apples with
pears"...
- Principle of optimization
In activities accompanied by ionizing irradiation, it is
necessary to observe such a level of radiation protection
that the risk of harmful effects is optimally low,
as far as can be reasonably achieved in
terms of application objectives, technical and economic
possibilities.
This
principle of optimization of radiation exposure is
sometimes referred to by the abbreviation ALARA
("As Low As Reasonably Achievable"), achieving
such low doses as are appropriate to the objective
possibilities and needs. The principle of optimization is
a very important and reasonable "middle way"
between underestimating risk on the one hand and
unjustified hysterical demands (often stemming from
purposefully induced radiophobia) on the other hand to
ensure absolute protection *) and zero doses, whatever it
costs, regardless of possible counterproductiveness even
in terms of the declared goals. Directing technical and
economic resources to appropriate health and social
programs can significantly improve people's health and
quality of life (or even save more lives), than
resources directed to excessive, ineffective and
irrational radiation protection!
*) This maximalist
approach is sometimes abbreviated as ALAPA
("As Low As Possible Achievable") - reaching
the lowest doses possible. In addition to radiation
protection, ALAPA abbrevation can also be found in a
number of other areas of medicine and technology,
sometimes as "As Low As Practically Achievable"
or "As Low As Practically Applicable" (omitting
other meanings of this word, eg alapa =
introductory melodic part of the Indian
rag ...).
In radiation diagnostics (X-ray, scintigraphy), this
means that image data are obtained in sufficient
quality at what the lowest
radiation dose.
- Principle of limitation
In activities with ionizing radiation, it is necessary to
limit the exposure of persons so that
the total radiation dose for certain periods (usually 1
year and 5 years) does not exceed the set limits.
The specific values of the limits
and their relation to the actual radiation risk are
constantly evolving. The limits were
introduced in 1956, a certain milestone was the ICRP
recommendation of 1977. Several types of exposure limits
have been introduced - general limits for the population,
limits for radiation workers, limits for
pregnant women. In technical applications, some derived
limits are also introduced (eg limits of
wastewater activity), the observance of which guarantees
that the limits of radiation doses for persons under
defined conditions are not exceeded. In general, we
cannot consider the limits as values separating radiation
safety from radiation damage, but as a boundary above
which the risk of stochastic effects from irradiation is
no longer acceptable. Some specific values of radiation
limits are given below (section "Basic methods of
radiation protection", section "Radiation
dose limits").
These general principles of
radiation protection have their specific form in medical
applications of ionizing radiation, see §5.7 "Radiation
protection in radiation diagnosis and therapy".
Part of radiation
protection is also ensuring the physical safety
of sources of ionizing radiation, which should be secured so that
there can be no uncontrolled irradiation or contamination of the
environment - so that the sources are properly stored and
registered, so that the source is not lost or stolen, so that
sources of radiation are entrusted only to persons and
organizations, that are trained and authorized for the relevant
activities.
Basic
methods of radiation protection
The task of radiation protection is to reduce the
absorbed dose of ionizing radiation in the organism to
the lowest possible level (reasonably
achievable - "ALARA") and thus
significantly reduce the risk of adverse
deterministic or stochastic effects of radiation. The obtained
radiation dose is
determined by several basic factors: the intensity, type and energy of the emitted
radiation we work with, the exposure time and the geometric
conditions (distance, shielding). So there
are three basic ways
to protect against external ionizing radiation (+ the fourth way
when working with open emitters) :
- 1. Time
The absorbed radiation dose is directly proportional to the exposure time during which we are in
the radiation field. By
shortening the time of stay in the exposed place, we can
reduce the received radiation dose proportionally. So we
do not stay unnecessarily long in an area with ionizing
radiation, and work with radioactive substances must be
thoughtfully prepared and carried out as quickly as
possible.
- 2. Distance
The intensity
of radiation and thus the dose rate are inversely
proportional to the square
of the distance from the radiation source (this applies exactly to the point source). It is therefore
necessary to stay as far
away
from radiation sources (ie also from patients with
applied activity), when working with radiators it is
useful to keep them as far away from the body and
possibly use suitable manipulators, tweezers, etc.
- 3. Shielding
A very effective protection is the shielding of radiation
with a suitable absorbing material. As has been shown from a physical point of view
in §1.6 "Ionizing radiation", section "Absorption of radiation in substances", the interaction of radiation with the
substance environment leads to the absorption of a
certain amount of radiation (sometimes all radiation) and
thus to attenuation of radiation flow.
Thus, if we place in the way of radiation a suitable shielding
material, we can achieve a significant reduction
in the intensity of radiation, sometimes even complete
absorption of radiation. The shielding should be as
close as possible to the radiation source, or
surround it (eg a bottle of
radioactive material is placed in a lead container). For each type of radiation, shielding is
performed in the following ways :
Gamma and X radiation
shielding
For gamma and
X radiation, the most suitable shielding materials are substances with high specific weight (density) - mainly lead, from building material then
concrete with possible admixture of barite and
the like. Lead (occasionally also tungsten) containers
are used for the transport and storage of radiators, further lead sheets, shaped lead bricks,
etc. For effective shielding of
gamma radiation with an energy of approx. 100keV a layer
of lead 2mm thick is enough; the higher the energy of the
gamma radiation photons, the thicker the shielding layer
must be used. If is the need to maintain the
optical visibility, used lead
glass with a high content of lead oxide in the
melt.
The thickness of the shield required
depends on the density (and nucleon number) of the
shielding material, the radiation energy g and the attenuation required. The
tables sometimes show the values of the so-called half-layer absorption, which is a thickness
of the shielding material which reduces the intensity of
the radiation by half (2 half
layers then 1/4 , 3 half layers to 1/8 etc. - shielding effect
increases exponentially with the thickness of the shield
according to the formula in §1.6). The absorption
half-thicknesses for various energies and materials are
given in the table in the section "Radiation absorption in substances" of §1.6.
Beta radiation shielding
For radiation shielding b- the light materials (such as plexiglass)
with a thickness of approx. 5-10 mm are sufficient,
preferably in combination with the subsequent a thin layer of lead to shield the braking
electromagnetic radiation generated
by the braking of electrons b in a light
shielding material. Lead alone
is not a suitable shielding material for radiation b, as it
produces hard and intense braking radiation, which would
require an unnecessarily thick layer of lead to shield it.
To shield positron radiation b+, in addition to a layer of light material, it
is necessary to use relatively thick layers of lead (at
least approx. 3 cm) in order to shield hard gamma
radiation with an energy of 511keV, generated at the
annihilation of positrons b+ with electrons e-.
Alpha radiation shielding
Radiation a, due to its low penetration,
can be shielded very easily. A thin layer (millimeter) of
light material, such as plastic, is enough. It is often
not necessary to shield against alpha radiation at all,
because even in the air there is a range of particles a only a few
centimeters, at higher energies tens of centimeters. If
the emitter is combined a + g, the gamma
shield automatically completely shields even the alpha
radiation as well.
Neutron radiation shielding
Shielding against neutrons is generally a more
complex problem than agains beta electrons or gamma
photons. If it is fast neutrons, they
must first be slowed down so that they
can be efficiently absorbed by a suitable absorber.
Neutrons are most effectively slowed down by the passage
of hydrogen-rich substances, where they lose energy
during elastic scattering on hydrogen nuclei (protons).
To reduce the number of fast neutrons about 10 times, a
layer of about 20 cm of paraffin or plastic is needed.
For the absorption of such slowed neutrons, their capture
by suitable atomic nuclei is then used. The most
effective absorption takes place in cadmium, boron or
indium. The absorption of neutrons in the nuclei of
cadmium or boron is accompanied by the emission of gamma
radiation (these are reactions (n, g) of
neutron radiation capture), which also needs to be
shielded by a heavy material - lead. Thus, neutron
shielding generally must consist of three layers:
a layer of light hydrogen-rich material (e.g.,
polyethylene), a layer of cadmium or boron, and finally a
lead layer.
Note: When shielding
neutrons, it is necessary to keep in mind that radionuclides
are formed during the capture of neutrons in some nuclei,
when originally inactive materials can become emitters b and g . These
radionuclides then "internally" contaminate
shielding and construction materials. E.g. if
cobalt-alloyed steel 59Co is exposed to neutrons, the known
radionuclide 60Co with a half-life of over 5 years is formed by
neutron capture !
- 4. Prevent of
contamination
When working with
open radionuclides (in the form of
solutions, powders, aerosols or gases), the risk of external
radiation is further
approched by the risk of contamination with radioactive
substances (§5.5, section "Radioactive contamination") - there may be surface contamination of the body, and
internal contamination. Internal contamination is
the most dangerous, because the organism is exposed to
radiation for a long time and "from the inside"
- the radionuclide enters
the metabolism and, depending on its chemical nature, can
accumulate in certain "target"
organs, which are then directly exposed
to the effects of radiation (the
previous three methods of protection are then irrelevant
here!).
Internal contamination can occur through the digestive
tract, respiratory tract, or skin penetration. To prevent
contamination, it is therefore necessary to follow the
general rules of hygiene, do not eat in the
controlled area, use protective gloves, work with
volatile radioactive substances in the fume hood, etc.
The issue of
internal contamination will be discussed in more detail
in §5.5, passage "Internal contamination".
| Unjustified
radiophobia when working with radiation : |
| With erudite work with
knowledge of the matter and adherence to the principles
of radiation protection, it can be
achieved that working with ionizing radiation is no
more dangerous and harmful than working with any
other materials, machines and equipment . |
The practical implementation of the
above principles of radiation protection can be significantly
aided by the use of protective aids in individual work
operations with ionizing radiation. On the one hand, they are shielding
aids - lead cases, covers, containers for radiators,
shielding walls, aprons, glasses, etc. Furthermore, handling
aids - tweezers, pipettes, remote manipulators,
conveyors, etc. When working with open emitters, they are aids
against contamination - rubber gloves, coats, veils,
airtight shoes and more.
Radiation dose limits
Any dose of ionizing radiation can be associated with a certain
risk of harmful effects (at least according
to the linear threshold-free hypothesis...), so care must be taken to keep the doses
as low as possible. For the purposes of assessing and controlling
radiation exposure, certain dose tresholds *) have been set per year and
for 5 years - the so-called limits (maximum permissible doses) for
workers with ionizing radiation sources, which are still
associated with a very low probability of radiation damage. The
current value of the annual limit
for workers
was set at 50 mSv, the five-year limit at 100 mSv (5 consecutive years). Basic
limits for other populations are set at 1 mSv/year
.
*) These are additional
doses to doses from natural sources. Spontaneous radiation dose
from natural sources are not included in the
limits for population or for occupational exposure of radiation
workers (however, they are included in
cases of targeted and professional activities associated with
increased exposure from natural sources - such as work in uranium
mines or in high-alitude aircraft).
5.4.
Radiation monitoring and personal dosimetry
Radiation monitoring is a targeted measurement
of quantities characterizing radiation in order to ensure the
optimal level of protection of persons and the workplace or
environment from the harmful effects of ionizing radiation.
Monitoring is performed at workplaces with ionizing radiation and
possibly even in the vicinity of more significant sources of
ionizing radiation. The basic quantities measured during
radiation monitoring are the radiation dose and
dose rate. Their direct measurement is performed using so-called dosimeters,
which are specially modified radiometers, calibrated in dose
units (Gray, Sievert). In addition, derived dose
determination methods are used based on other quantities, eg
radiation intensity or radionuclide activity.
Reference levels
For the evaluation of measurement results during monitoring,
certain significant values are determined, the achievement of
which signals a certain anomalous radiation situation and is, if
necessary, an instruction to initiate appropriate radiation
protection measures. Three types of reference levels are
introduced :
- Recording level
This level determines the lowest value
of the monitored quantity, from which it is has meaning
to evaluate it and record it in the documentation. The
smallest detectable value of the measured quantity or the
background value is usually taken as the recording level.
This value depends on the type of measured quantity,
specific measurement conditions and properties of
measuring instruments used for monitoring.
- Investigation
level
Achieving the investigation level is already a symptom of
a not entirely normal radiation situation in the
workplace and should be an incentive to investigate
its causes and consequences. The examination level is
usually set as the upper limit of usually occurring
values, and in the case of personal radiation doses,
optionally as 0.3 times the relevant limit for radiation
workers.
- Intervention level
Achieving this level already signals an
extraordinaly event or radiation accident,
associated with increased radiation risk, and is an
incentive to immediately warn and take steps to protect
people and the environment according to the workplace
emergency regulations.
Radiation monitoring usually consists of three
parts: monitoring of persons, monitoring of the workplace,
monitoring of radioactive waste and possibly monitoring of the
surroundings of the workplace with ionizing radiation.
Personal
monitoring - personal dosimetry
Personal monitoring consists in measuring the personal
radiation doses of individual radiation workers, whether
it is external exposure, or possibly on internal
irradiation from radioactive contamination. Monitoring
of external radiation is performed using personal
dosimeters, which radiation workers wear during all work
with ionizing radiation and staying in the controlled zone. These
dosimeters are centrally evaluated at specified time intervals
(usually 1 month), the result is dose values (in mSv).
Note: It should be noted that direct
measurement of the absorbed dose is very difficult, apart from
laboratory methods, generally dosimetric quantities are
not directly measurable. Doses are determined indirectly
and approximately, based on certain model assumptions. The
surface dose and the dose at a certain depth are derived from the
data on the dosimeters. The most complex is the dosimetric
analysis of internal contamination - it is described in
more detail below in the section "Internal
contamination".
The following types of personal dosimeters
are used :
- Personal film
dosimeters
Film dosimeters are based on the photochemical effects of
ionizing radiation (see §2.2
"Photographic
detection of ionizing radiation"). The basis of the
film dosimeter is a small field of photographic
film, light-tightly wrapped in black paper (it differs from ordinary photographic film in
that it has a thicker emulsion with a higher content of
silver bromide). The ionizing
radiation passes through the film coating and creates a
latent image in the photoemulsion, which is visible after
development. The optical density of the graying or
blackening of the film, which can be evaluated
photometrically, is then a measure of the integral
amount of radiation which passed through the
film during the exposition; it also indicates the dose
of radiation that would be absorbed in the
tissue irradiated during this exposure.
The frame of film is inserted into
a plastic case (Fig.5.4.1 above),
provided with several small rectangles of copper and lead
plates of different thicknesses, which serve as filters
absorbing radiation g
depending on its energy. These
filters are used both to correct the dependence of
blackening on the energy of radiation, and on the other
hand by comparing blackening under an unshielded surface
and under individual filters it is possible to estimate
the type and roughly the energy of radiation (of course, the film itself does not have
spectrometric properties). The film
dosimeter is worn by workers at the reference point (left pocket on the shirt) and
is regularly (once a month) changed, developed and photometrically
evaluated; using a suitable calibration factor,
the resulting measured value is the effective
dose in mSv.
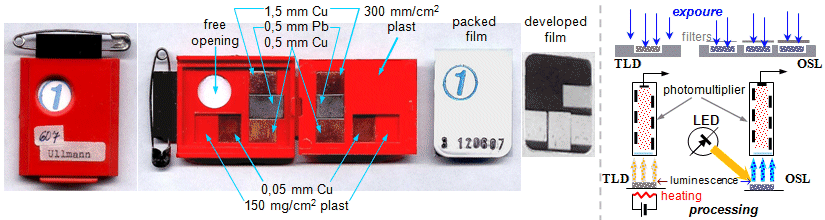
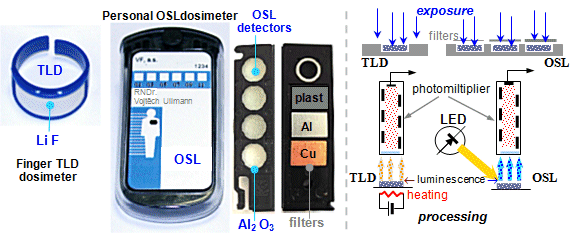
Fig.5.4.1. Above: Film personal
dosimeter. Bottom:
Thermoluminescent (TLD) and optical-luminescent (OSL)
dosimeters.
- Thermoluminescence
dosimeters, OSL dosimeters
(Fig.5.4.1 below) - are described in §2.2 "Photographic
detection of ionizing radiation", section "Thermoluminescence
and photoluminescence (OSL) dosimetry".
- Personal neutron
dosimeters
- Electronic
dosimeters with direct
reading
A certain disadvantage of the above-mentioned integral
personal dosimeters is the time retardation
- we will find out the received dose only after the
central evaluation of the personal dosimeter, usually
after a few weeks. Electronic dosimeters
*) are used for immediate and continuous measurement of
personal radiation dose - see §2.1 "Methodology of
ionizing radiation detection",
Fig.2.1.1. These devices contain a suitable electronic
radiation detector (usually a G.-M. tube, for higher
doses event. an ionization chamber) and evaluation
electronics with a numerical display for immediate
reading. They allow to measure both the instantaneous
dose rate and the total (summary) dose. It is also
usually possible to set a certain signal level
of the dose rate or dose, above which an acoustic
indication is triggered. Thanks to the miniature
dimensions, they can be worn similarly to other
personal dosimeters.
*) These dosimeters have replaced
previously used pencil dosimeters with
an ionization chamber and an electrometer, the fiber hand
of which allowed immediate reading of the dose.
Radiation monitoring of the
workplaces and environment
In order to ensure the optimal level of radiation protection
and radiation prevention, it is important to monitor the
intensity of ionizing radiation and the possible presence of
radioactive contamination in the areas of workplaces, their
surroundings and in the environment of the population - the
so-called radiation monitoring. Measuring
dose and dose rate are performed in laboratories,
examination rooms and nuclear medicine departments. A system of
workplace monitoring program is established.
.......................
5.5.
Open radionuclides. External and internal contamination
Closed
and open emitters
In terms of material nature and technical design, sources of
ionizing radiation are divided into two groups :
- Closed emitter
is a radioactive emitter whose construction ensures (tested and certified *) tightness and excludes the
release of radioactive substances into the environment
under the anticipated conditions of use and wear. Closed
emitters are, for example, various standards for the calibration of
measuring instruments, radioisotope irradiators in
radiotherapy, radiation sources in industry (eg in
defectoscopy).
*) The actual tightness of the
closure of the radioactive source must be regularly
verified by wiping tests, or testing the
long-term stability of radioactivity (possible
differences from the correction for decay).
In the
category of closed emitters can,
in a sense, also
be included "automatically" the electronic sources of radiation
- X-ray devices or accelerators, where there are no
radioactive substances.
Note:
However, for higher energy accelerators, the situation
may be more complicated, as such "closed
sources" may activate originally
non-radioactive substances by nuclear reactions (§1.3 "Nuclear reactions") and thus create
open emitters.
Radiation protection when
using closed emitters consists in protection
against external radiation.
- Open emitter
does not meet these conditions for a closed emitter -
they are mainly radioactive
solutions, gases, aerosols, powders, etc.,
which can be divided, portioned or otherwise
modified. In nuclear medicine, all radioactive
preparations for in vivo and in vitro
examination, as well as for radioisotope therapy, are
open emitters.
Compared to closed
emitters, radiation protection is more
demanding with open emitters - there can be
both direct external
irradiation with ionizing radiation (as with
closed emitters) and also subsequent irradiation
due to radioactive contamination, external or internal.
During internal
contamination, when a radionuclide enters the
internal environment of the organism, direct irradiation
of tissues and organs occurs, from the immediate
vicinity, often at the cellular level.
Some
radioactive substances are rapidly excreted from the body
during metabolism, others bind to the target tissues and
organs for a long time in the body. Once a radionuclide
enters the body, it cannot be protected
from (internal) radiationin the above classic ways -
time, distance and shielding. To a limited extent, some
substances can be used that accelerate the metabolism and
excretion of the radioactive substance (radionuclide in
the appropriate chemical form) from the body. The issue
of internal contamination is discussed below in the
section "Internal contamination").
For the classification of emitters
according to activity in relation to radiohygienic risks, the
so-called acquit level of activity is
introduced: it is a value of total activity (or specific mass
activity), below which radiation risks and radioactive
contamination are considered negligible (compared to natural
sources of radiation). Such an emitter is not subject to
radiation protection regulations and we could have it without
risk, with a bit of exaggeration, even at home in the living
room...
According
to the severity of radiation risk, sources of ionizing radiation
are divided into 5 categories :
Insignificant sources
(eg small closed standards for spectrometric calibration,
ionization fire detectors, radioactive substances with activity
lower than the acquit level,
etc.);
Small sources
(such as stronger closed emitters and low activities open sources);
Simple sources
(eg X-ray diagnostic devices and defectoscopic devices);
Significant sources
(eg closed emitters for radiotherapy, accelerators, highly active
open emitters); and finally
Very significant sources
(such as nuclear reactors or radionuclide production facilities).
Laboratory work with open radionuclides
and their storage, radioactive waste, application of
radionuclides to patients.
Radiation hygiene when using open emitters -
protection of workers from external radiation and internal
contamination .
Radioactive
contamination
When handling open radioactive substances, their leakage and
subsequent contamination (pollution) of objects, the working
environment and persons with these radioactive substances can
occur. Contamination can be surface external and internal.
Surface contamination
Surface contamination of work surfaces, aids, clothing or people is most
common. Surface contamination of people can lead to higher
doses of radiation, especially on contaminated areas of the
skin, but in some cases can also result in subsequent
internal
contamination, see below. For continuous control of surface
contamination during and after work, radiometers with large-area probes are used,
which should be located in all exposed workplaces and in hygienic
loops.
A sensitive method of contamination control is the method
of wiping from a
defined possibly contaminated area of
the exposed site with a
cotton swab soaked in a
suitable solvent (alcohol-gasoline)
and then measure it in a test tube with a well scintillation
detector.
Decontamination
In the event of contamination of the working environment, it is necessary to prevent the spread of contamination, mark a visibly contaminated area, report this incident to a specialist and cooperate in decontamination under his guidance. When decontaminating,
it is necessary to first aspirate as
much of the active
liquid as possible with filter paper or pulp and then wash and wipe the contaminated area with a suitable
cleaning or decontamination agent. Generated waste it is necessary to store in plastic bags
and contaminated objects decontaminated
or store them in
plastic bags for decay of radioactivity.
Contaminated water must be poured into the waste of decay
sumps. The effectiveness of decontamination is continuously
checked by measuring with a radiometer. If the radioactivity
cannot be completely eliminated, the site should be marked and
covered with protective paper or foil; the expert or
supervisor will then
decide on the further procedure and resumption of operation.
In the event of personal contamination, the worker must remove contaminated
clothing or protective equipment, check the contamination of the
body surface and, if necessary, clean
it by washing or showering. It is also necessary to check whether
there has been any internal
contamination of the worker. In cases of suspected internal
contamination and exceeding the maximum permissible dose of
radiation is necessary to take the medical
and radiohygienic measures, including the
temporary exclusion of the the worker from the environment with
ionizing radiation.
Extensive
radioactive contamination is already a radiation accident
- see the following §5.6.
Internal contamination
When handling higher activities of open emitters, unwanted
penetration of radioactive substances into the body can occur
- internal contamination and subsequent internal
irradiation.
Note: A
special case of "internal contamination" is the
deliberate application of a radioactive
substance - a radioindicator, radiopharmaceutical
- to an organism for the purpose of diagnosis or
therapy in nuclear medicine ("Scintigraphy", "Radioisotope Therapy"). From
a radiohygienic point of view, this is not considered
as contamination.
Upon penetration into the body, the
radioactive substance enters the metabolism and can be distributed
in individual tissues and organs depending on their chemical
composition - part accumulates in the so-called target
organs, the rest is distributed throughout the body.
Most of the radioactivity is then metabolized
and after a certain time it leaves the body (mostly in the urine, to a lesser extent in the faeces,
sometimes in sweat or exhaled air).
However, part of the radioactivity may remain permanently
bound (eg iodine in the thyroid gland, strontium or
plutonium in the bones, cesium in the muscles, ...) - it is retention
(Lat. retineo, retentum = hold back,
retain). Due to inhomogeneous
distribution of radioactive substances in target tissues and
organs, the internal irradiation of the organism may be highly heterogeneous
(see below "Determination of the radiation dose from
internal contamination. MIRD method").
Radioactive contamination can enter the
organism in basically four ways :
- Ingestion
The most common cause of internal contamination,
especially in laboratory work, is ingestion
of radioactive material through contaminated hands or
other objects that come into contact with the mouth.
- Inhalation
When working with radioactive gases, vapors or aerosols,
radioactivity can penetrate when inhation
into the lungs, and from there possibly through the walls
of the alveoli into the blood and further into the
organism.
- Through the skin
When contaminating the surface of the body, some
substances are able to diffuse and penetrate the body
even through intact skin (eg plutonium
or phosphorus), or by an open skin injury.
- Targeted
administration of a
radiopharmaceutical,
mostly intravenous or oral - for diagnosis or therapy in
nuclear medicine ("Scintigraphy", "Radioisotope therapy").
When a radioactive substance enters the body
through ingestion or inhalation, the substance passes relatively
quickly from the digestive tract or lungs to the blood and lymph.
Its other behavior - distribution, metabolism, persistence or
excretion from the organism - is determined by the chemical
properties of the substance and the rate of radioactive
conversion of the radionuclide.
Monitoring of internal
contamination
Determination of internal contamination with g -radionuclides can
be performed by external
measurement of gamma radiation using a sensitive scintillation
detector above the critical (target) organs. E.g. in 131I it is a thyroid gland, so in
workplaces performing thyroid therapy with radioiodine,
it is necessary to
periodically measure the 131I activity of the thyroid gland in
all workers involved in these therapies.
A special method of measuring internal contamination is
the use of whole-body detectors of
radiation, equipped with scintillation or semiconductor
detectors, which are installed in some workplaces with high
radioactivity (nuclear reactors, production of radionuclides) and
risks of internal contamination - category IV. workplaces. These
methods of external monitoring are mainly used
for internal contamination by radionuclides emitting g radiation, which
penetrates through tissues out of the body. For pure emitters b, external
monitoring can only be used if, due to the high energy, the
electrons b generate harder braking radiation in the tissue (eg 32P or 90Sr- 90Y), which penetrates
through tissue out of the body. For low energy radiators b it has low
intensity and energy braking radiation, it does not penetrate out
of the body and external detection cannot be used. External
detection is, of course, not applicable to pure alpha emitters.
Another method of monitoring internal
contamination is to measure the activity of
blood and urine samples.
Determination
of radiation dose from internal contamination. MIRD method.
During external irradiation of the organism, the energy
of radiation is transferred to the tissues and organs only for
the time when the body is exposed to radiation. If the
radionuclide enters the body, it remains deposited there
and irradiates it for a long time. Irradiation is heterogeneous
and changes over time, as the radionuclide moves and distributes
in the body, is excreted from it, and how decreases it amount by
radioactive decay. It is generally a complex function of
time and space.
Accurate determination of radiation doses
in individual tissues and organs during internal radioactive
contamination is therefore generally a very complex and difficult
task. The absorbed dose depends not only on the physical
characteristics of radionuclide (types and energies of
emitted radiation, half-life), but also on its chemical
form (and very significantly!), normal or pathological pharmacokinetics
- rates of accumulation and excretion of the substance in organs,
on anatomical factors (sizes, distribution and
densities of organs and tissues).
A more detailed analysis of dose
distribution in tissues and organs will be provided below. In
terms of risk for stochastic effects, for approximate and global
assessment of the radiation load from internal contamination are
use so called conversion factor of radiological
contamination h [Sv/Bq]: it is a coefficient indicating the effective
dose in the body (ie. cummulative time duration
effective dose), caused by the uptake of a
unit activity of 1Bq (in practice 1MBq) of a given radioactive
substance into the organism. The values of the conversion factor
depend not only on the type of radionuclide, but also on the
compound in which the radionuclide is present and on the way in
which the radionuclide has entered the organism; values for ingestion
hing and
for inhalation hinh are most often given. Conversion factors are given for
the so-called "reference person" with
anatomical and physiological characteristics typical of the
average population, eg weight 70 kg. More accurate dose values
from internal contamination are determined based on the following
models of MIRD metrod :
Radiation dose D
[Gy] in the organ in which the radioactive substance is contained
(assuming an even distribution for simplicity) is given by the
product of two quantities
D
= S . A S :
¨ The accumulated
activity of AS
indicates the total number of radioactive transformations that
take place in the considered organ during the entire presence of
the radionuclide (for the whole time). The value of accumulated
activity AS is determined as an integral or area under the curve of
time dependence of activity A(t) in the organ from time t=0 of
radionuclide entry, to its complete disappearance (theoretically
up ¥):
AS = 0 ò¥ A(t) dt (middle part Fig.5.5.1).
The excretion of a given
radioactive substance from the organ (or the whole body) has an
approximately exponential time dependence and
its rate is characterized by the so-called effective
half-life T1/2ef . This effective half-life is is given
by a combination of the physical half-life T1/2phys of a
given radionuclide and the so-called biological half life
T1/2biol , i.e. the time required to eliminate half of the
absorbed amount of a given substance by metabolism. The effective
half-life is then T1/2ef = T1/2biol .T1/2phys/ (T1/2biol+T1/2phys). For the kinetics of radionuclides in tissues and
organs, the biological half-life of excretion is usually
dominant, which is usually significantly shorter than the decay
half-life of the radionuclide itself.
¨ The dose constant S
represents the dose related to the unit activity of a given
radionuclide in the organ [Gy/Bq] (in
practice [mGy/MBq]). The constant S
includes the influence of all types of radiation emitted by a
given radionuclide - particles b and a, radiation g, characteristic
and braking X-rays, conversion and Auger electrons.
Alpha and beta particles (as well as
conversion and Auger electrons) are completely absorbed in the
source organ and contribute only to the dose in this organ, which
in this case is D = <Ea,b>. AS /m, where <Ea,b> is the mean energy of the emitted particles a or b, AS is the
accumulated activity, m is the mass of the organ or tissue
(the dose constant is therefore S = <Ea,b >/m in this
case). The basic relation for the radiation dose from the
homogeneous distribution of radioactivity in the substance was
derived above in §5.1, passage "Radiation dose from radioactivity".
Penetrating gamma and X radiation, in
addition, can also shine through into the surrounding tissues and
organs (Fig.5.5.1 on the left). Its contribution to the constant S
depends on the energy of the radiation, the distances, the size
and shape of the source and target organs, and partly on the type
of tissue between them.
Dose constant values for
individual organs and radionuclides are determined using
microdosimetric methods including phantom modeling and
simulation; in the simplified version, they are given in the
radiation protection tables (these are average values based on a
person weighing 70 kg).

Fig.5.5.1. Radiation doses from the distribution of radioactivity
inside the organism. Left: Source and
target organs in the body. Middle: Time
dependence of activity in source organs. Right:
Time dependence for determination of doses by MIRD method.
<E> is the mean energy [J] deposited in the tissue of
weight m per one decay of the used radionuclide (beta electrons or alpha particles are considered). If this mean energy is given in nuclear units [eV],
there a conversion factor of 10-19 is also used.
The figure roughly simulates the situation
after the penetration of radioiodine 131I into the organism. Radioiodine is rapidly taken up in
the thyroid gland, then metabolized and excreted by the kidneys
into the bladder, from where it periodically leaves the body
during urination.
The tissue or organ in which we
need to determine the radiation dose is called the target,
other organs and tissues in the body are considered to be the source
for it - the left part Fig.5.5.1. The radiation dose DT in target organ T
is the sum of doses from the "own"
accumulated activity AST contained in this
organ and the dose contributions of penetrating
radiation from the activities ASi accumulated in the surrounding source organs
"i" :
D
T
= ST . AST + i = 1SN (Si .AS i) ,
where Si
are the dose constants for target organ irradiation from activity
AS i contained in the
surrounding organs "i" (which are the source for target
organ T; in general, each organ can be considered as both
a target and source for other organs). The constants Si include, inter alia,
the absorption of radiation in tissues and the decrease in
radiation intensity with distance. In the first approximation,
the proportionality Si ~ e - m .d
/d2 applies, where m(Eg , r) is the linear attenuation factor of tissue density r for radiation of
energy Eg , d is the distance of the source organ from the
target organ. However, the precise calculation should include
integration across the entire space between the source and target
organs, as well as across the actual volume of both organs.
This complex method, including the
above-mentioned physical and biological factors for the
determination of radiation doses in individual organs from the
internal distribution of radioactive substances, is called MIRD
(Medical Internal Radiation
Dose). The basic MIRD method uses some flat-rate
data on anatomical conditions in the so-called "standard
human" weighing 70 kg. The refinement of these data can be
achieved by using volummetry on density images
from X-ray CT - the so-called voxel method. And
the specification of actual data on the distribution and
pharmacokinetics of a given radioactive substance can in
principle be obtained by means of quantitative
scintigraphy.
Such an improved, so far the most complex
and most accurate method of determining radiation doses in organs
and tissues in 3D radiopharmaceutical dosimetry, is
important in planning and monitoring the course of radionuclide
therapy in nuclear medicine - a more detailed analysis is given
in the part "Radioisotope therapy" of chapter 3 "Application of ionizing
radiation". It should be point out, that due to the
significant variability of biological factors in particular, even
when the inclusion these complex methods mentioned above, can
not achieve a high accuracy determination of radiation
doses in organs - the error is about 30% or above..!..
In practice, for the resulting radiation
dose in the target organ, the dose from the organ's own
accumulated radioactivity is dominant, while the contribution of
radiation from other more distant source organs is usually
negligible. This is especially true when the radioactive
substance is a pure emitter b
(or a), or the proportion of penetrating g-radiation is not
high. And so it is in the vast majority of cases of
radiation-significant internal contamination, including the
application of radiopharmaceuticals in nuclear medicine.
5.6.
Radiation protection at workplaces with ionizing radiation
(Categories of workplaces, controlled zone, storage and disposal
of radioactive waste.)
Organization
of workplaces and their categories
The construction, layout and equipment of workplaces must be
carried out in such a way as to ensure sufficient radiation protection of workers, other persons and the
environment. In the event of an accident, it must be possible to
decontaminate people and the workplace as quickly and efficiently
as possible.
Workplaces are divided according
to whether they are designed to work with closed emitters (such
as X-ray or radiotherapy
workplaces) or with open emitters. According to the severity of
the radiation risk, workplaces are divided into 4 categories, for
workplaces with open emitters it is according to the processed radioactivities.
¨ Category I.
workplaces
they are for working with low activities of radionuclides with low radiotoxicity (small sources of IR) and in terms of construction and equipment does not differ from
conventional chemical laboratories.
¨ Category II workplaces
process medium activities of open radionuclides, they have a controlled
zone and are equipped with
protective equipment incl. hoods, or there is a separate sewer
for radioactive waste.
¨ Category III workplace
is designed for demanding work with strong sealed emitters
(accelerators, irradiators in radiotherapy and industry) and with
high activities of open radionuclides (eg radioiodine therapy,
uranium ore mining and processing, radiochemical plants).
Therefore, considerable requirements are placed on its
construction and equipment in order to ensure the fastest and
most effective cleaning in the event of contamination. There
should be 3 types of rooms in the controlled area: for demanding work with high radioactivities ("hot" operations),
for routine laboratory
work and measuring rooms. In addition, a special room or areas for storage of
radionuclides and
radioactive wastes. Floors and walls of laboratories must be
smooth and washable, floors are further sloped and provided with
waste. Intensive ventilation with active air filtration with an
outlet above the roof should also be provided. Liquid radioactive waste is led to decay
tanks. The workplace must
be equipped with suitable shielding, manipulators, fume hoods and
devices for protective dosimetry. The controlled zone should be separated
from other areas by hygienic
loops with a radiometer for
checking of contamination and
with the washroom.
¨ Category IV workplaces
This most risk category includes nuclear reactor operations, radionuclide production and
radioactive waste repositories with high activities and long half
- lives. In addition to irradiation and contamination directly at
the workplace, there is also a risk of environmental
contamination, which
implies the need for radiation monitoring of
the workplace
and its surroudings.
Controlled zone
The radiohygienically controlled zone is called those areas of the
workplace, where ionizing radiation (radioactive
substances or other sources of ionizing radiation) is
handled and where the regime of protection of persons
against ionizing radiation must be observed *). Entrances to the controlled area must be marked with warning signs. Only trained
radiation workers with protective equipment and personal dosimeters have free access there, other
persons only accompanied by radiation workers and their stay is registered.
*) The current
radiation protection standards
specify:
"Controlled zone is defined wherever it is expected that
during normal operation or with foreseeable deviations from
normal operation, the radiation dose of workers could exceed 3/10
of the limit for radiation workers".
In some workplaces, especially I. category, the so-called monitored
zone is being introduced,
which is an area where, during normal operation of radiation
sources, the radiation dose could exceed the general limits for
the population.
Radiation
accidents and crashs
In every human activity - in industry, transport, agriculture,
health, science and technology, laboratory work, as well as in
everyday life, sometimes something "fails", breaks,
will spoil - an accident occurs. Of course, this can also
happen in workplaces with ionizing radiation.
By radiation
accident we
mean an unplanned event that will increase the danger of people from ionizing radiation. In workplaces with closed emitters, it is mainly about
unwanted exposure of people. In workplaces with open emitters,
it is mainly an uncontrolled leakage
of radioactive substance into the working environment (eg by
spilling, spraying, breaking the bottle with the radioactive
solution, etc.) with subsequent contamination
of the workplace,
environment or workers. Such events can occur when handling open
emitters in the process of their preparation, transport, storage,
application and disposal.
For radiation accidents (especially minor
ones), the name extraordinary event is sometimes
used. The extent of a radiation accident or emergency is
distinguished by the 1st to 3rd degree of severity
:
- Degree 1
A minor radiation accident or emergency that has a
limited and local impact, the usual
means of operating personnel are sufficient to solve it,
there are no deterministic effects of irradiation. An
example can be the spillage of a small amount of
radioactivity on the floor during laboratory handling.
- 2nd degree
This is a more serious irradiation or contamination
of the workplace, which, however, does not yet require
measures to protect the population and the environment,
and its management will suffice the resources of the
workplace, possibly in cooperation with other
professionals.
- Degree 3
This is a serious radiation accident
associated with the dangerous release of radioactive
substances into the environment, requiring the
introduction of measures to protect the
population and the environment. The most serious
radiation accident (3rd degree) is also referred to as a radiation
crash. In the event of severe radiation
accidents, lethal exposure to persons at
the accident site can also occur.
An international 7-level table
is also used to evaluate radiation accidents in nuclear reactors
: 1st stage = deviation from normal operation; 2nd degree =
fault; 3rd degree = serious disorder; 4th degree = accident with
effects in a nuclear installation; 5th degree = accident with
effects on the environment; Stage 6 = accident with serious
radioactive consequences; Level 7 = major crash with extensive
radioactive consequences.
Below we will
briefly mention some examples of more serious radiation
accidents.
Nuclear
accidents and disasters with open emitters
One of the typical situations where a serious radiation accident
with open emitters can occur, is careless work with fissile
material (especially uranium 235U or plutonium 239Pu), especially if it is in higher concentrations - it
is the so-called enriched. If a larger amount of such
material is available, the critical amount may
be exceeded and a chain fission reaction may be triggered,
producing a very strong flash of neutron radiation
and g radiation, followed by high contamination
with fission products. Persons at the scene of an
accident receive very high doses of radiation,
often lethal. Several accidents of this kind have happened in
laboratories and nuclear plants.
For example, there was an accident at the Tokai-Mura
nuclear enrichment plant in Japan on September 30, 1999. Here, 3
workers prepared nuclear material in a solution of uranium oxide
(enriched to more than 18% 235U) and nitric acid. Inadvertently, more uranium solution
was added to the reaction vat, resulting in a supercritical
amount. A bluish flash signaled the ignition of the chain
reaction. Two workers standing closest died, a third worker was
being treated for an acute radiation sickness.
A number of radiation accidents have
occurred at nuclear reactors. A moderate
accident (level 5) occurred on March 28, 1979 in Three Mile Island nuclear
power plant on River Island near Harrisburg, Pennsylvania in the USA. Due to a failure of the
secondary cooling circuit pump, the temperature and pressure in
the primary circuit increased, the pressure relief valve opened
and the reactor was stopped in an emergency. However, the safety
valve blocked in the open position, the pressure in the primary
circuit dropped, some spare water cooling pumps failed, the
remaining heat began to boil the water, and several fuel cells
were burst. Radioactive water, steam and gases leaked into the
surroundings - a wide area around the power plant was infested,
and several thousand people were evacuated. As later in
Chernobyl, the perpetrators kept the accident completely hidden
for several days; however, unlike Chernobyl, the circles
concerned later succeeded in the concealment of
real extent of the accident, so that reliable data on the amount
of radioactive material leaked are lacking.
Accident at Chernobyl
The most severe radiation accident to date (7th
degree) occurred on April 26, 1986 at the Chernobyl
nuclear power plant (the principle of operation of nuclear
reactors and their safety is discussed in §1.3 "Nuclear
reactions", section "Fission
of nuclear nuclei"; and the causes
of the Chernobyl accident are described in the section "Chernobyl nuclear reactor accident"). During the destruction of the nuclear reactor,
there was extensive contamination of the
environment with radioactive fission products and the exposure of
232 people with high doses of radiation (units
up to tens of Sv), associated with deterministic effects and
acute damage to health; in 31 cases there were even lethal
effects (of which 2 workers were killed directly when the reactor
exploded, but even if this did not happen, they would receive a
lethal dose of radiation)! Many hundreds more people received a
radiation dose of tens to hundreds of mSv, for which an increased
incidence of stochastic effects can be expected (by at least 1%;
although it has not yet been confirmed ...).
Fortunately, despite the seriousness and local
tragedy of the Chernobyl accident, its consequences turned out to
be significantly smaller than they initially
seemed. And, of course, many times smaller (at least 100 times
smaller!) than claimed by some propaganda materials, manipulated
for political reasons (Cold War -
anti-communism, anti-Sovietism), or from
the motives of particular interests (fight
against nuclear energy, coal lobby, etc.).
In any case, the Chernobyl accident has become a milestone
in nuclear energy and radiation protection. It led to a
substantial tightening of safety regulations and
standards of radiation protection not only in nuclear energy, but
in the whole area of ionizing radiation applications (this resulted also in excessive bureaucratization of
radiation protection - cf. the section "Bureaucratic
requirements for radiation protection" in §5.8). An accident as
large as in Chernobyl will probably never happen again
!
Another recent major nuclear
accident in Japan is briefly described in the section "Accidents at the Fukushima Nuclear
Power Plant". No one was to blame
for this accident, it was caused by a huge natural disaster. It
has also been formally rated 7, but this is misleading, as its
scope and severity are incomparably smaller than at Chernobyl.
In large radiation
accidents with extensive radioactive contamination of the
environment, the main health risks and injuries in the majority
of the affected population are often caused not so much by
radiation, as by stress from evacuation and concerns arising from
overestimation of risks, radiophobia ...
Radiation
accidents with closed emitters
Even with closed emitters, serious radiation
accidents can occur if their radiation intensity (dose rate) is
appropriately high. Potentially dangerous emitters from this
point of view are particularly strong radiotherapeutic
irradiators or industrial emitters, eg
for defectoscopy or sterilization. Careless handling of such
unprotected emitters can result in external exposure
of the body to high radiation doses either whole body
(Þ
radiation sickness - sometimes lethal, increased incidence of
stochastic effects) or locally (Þ radiation burns).
A tragic radiation accident of this kind
happened in September 1987 in the city of Goiania
in the Goias region of Brazil, where a cesium emitter 137Cs with an activity
of about 50,000 GBq was unprofessionally and uncontrollably
removed from a radiotherapy irradiation intended for disposal.
Ignorant workers took it home, dismantled it and then sold it for
scrap. The workers of the waste storage warehouse also
distributed the radiator and took its individual parts home (they
liked the bluish fluorescence!), even children played with them.
The result was 5 deaths from radiation sickness, 20 people had
local radiation burns (mostly on their hands).
Other radiation accidents, some with lethal
consequences, occurred during the theft of emitters. There are
even several known cases of criminal abuse of emitters
against persons (murder or attempted murder). The perpetrators
and victims of these radiation plots are mostly persons those
involved in espionage activities and organized crime.
Many radiation accidents have become overexposed
to patients during radiotherapy due to incorrect
calibration of the irradiator or an error in the irradiation
plan. A serious accident of this kind happened in December 1990
at the University Hospital in Zaragoza, Spain, where poor
calibration of the linear accelerator caused 2-7-fold
overexposure of irradiated patients, as a result of which 18
patients died from radiation and another 9 suffered radiation
damage.
It should be noted with
satisfaction that radiation accidents are relatively rare
at present. The field of applications of ionizing
radiation is monitored, coordinated and secured as thoroughly (sometimes perhaps overly bureaucratically...), like perhaps no other field of human activity. Mostly
professionally founded people work here, well acquainted with the
principles of working with radioactivity and ionizing radiation
as well as with the principles of radiation protection.
A radioactive contamination ("dirty")
bomb
This is the name given to a weapon in which a classic
explosive is combined with radioactive materials.
Its purpose is to radioactively contaminate the
surroundings around the explosion by scattering radioactive
substances. It consists of a chemical explosive charge with an
admixture (or casing)
of radioactive substances. The explosion of a conventional
explosive sprays radioactive substances into the surrounding
environment. The extent of contamination depends mainly on the
strength of the charge and the amount of radioactive material,
the place of detonation (height above the
ground), the surrounding terrain (including urban development), the
speed and direction of the wind. Contamination from a practically
feasible "dirty bomb" is usually dispersed only within
a few hundred meters or at most a few kilometers from the place
of explosion.
It can have biological effects of ionizing
radiation on people in the affected area (usually
not fatal), but the main intention is to
induce mass radiophobic panic. There are also large
economic costs for decontamination and losses from interruption
of production activity. In the case of this
radiation-contaminating terrorist attack, it is necessary to
carry out basically similar radiohygiene measures as in
radiation accidents of nuclear facilities ("Radiation
accidents and crashs",
3rd degree of seriousness of a radiation accident, or 6th degree
of a nuclear reactor accident).
Only some radionuclides, with a long
enough half-life, they are suitable for the purpose of a
radioactive contamination bomb. They are, for example, cesium-137, cobalt-60, iridium-192, strontium-90, plutonium-238, americium-241,
californium-252, or polonium-210, radium-226. And, of course,
the fission decay mixture of radionuclides in spent fuel
from nuclear reactors is highly effective (§1.3,
pasage "Fission products"). There is also the
question of the availability of a sufficiently large
amount (activity) of suitable radionuclides (e.g.
americium-241, or californium, is not available in large
quantities...).
The military use of the radioactive
contamination bomb is practically zero, but there have been
concerns that it could become a weapon in the hands of terrorists.
Fortunately, it is not too easy for them. Obtaining large
quantities of suitable radionuclides is difficult (on the black market, from lost or stolen sources in
industry, medicine, research; regular sources are strictly
registered). Handling highly radioactive
substances is dangerous, requires professional competence,
demanding laboratory conditions and radiation protection.
Individual terrorists don't stand a chance here, it could perhaps
be partially successful with larger terrorist organizations that
could pay for a team of nuclear experts. However, the real danger
is probably small..?..
Early detection and prevention of illegal
transport of radioactive materials and attempts to prepare
radiation-contaminating terrorist charges is basically possible
by radiometric monitoring using G.M, scintillation or
semiconductor detectors of ionizing radiation, especially gamma
radiation. These devices are installed at airports and border
crossings.
Radioactive waste
Radioactive waste is such an unusable
*) material (substance or object) generated during the production and use of
radiation sources, that contains radioactive substances.
According to the above classification of radioactive emitters,
these wastes are open
emitters.
Radioactive waste can be potentially dangerous for the environment - it can
cause unwanted irradiation or contamination.
*) However, this unusability may be relative and conditioned by the current
state of technology. With the development of new technologies,
initially difficult waste may become a welcome
raw material
(see, for example, the section "Nuclear
waste" in
§1.3).
How can
radioactivity be destroyed ?
It is known that almost all
pollutants can be eliminated by incineration -
at high temperatures above about 600 °C chemical bonds break
down, oxidation occurs and the substance ceases to be toxic (with some exceptions are heavy metal compounds, which
are sometimes difficult to convert to harmless compounds). This does not apply to radioactive substances - the
actual radioactivity
cannot be destroyed by incineration! -
see the passage "Independence of radioactive decay
on external conditions" in chap. 1.2 "Radioactivity". By normal incineration we only
break down or change the chemical bonds, but the number of
radioactive atoms remains exactly the same as before incineration
- some of them escape in gaseous form in smoke, some remain in
the solid phase in the ash. To destroy radioactive nuclei, we
would have to heat the substance to a temperature of several
million degrees for nuclear reactions to occur. In this difficult
way, we could hopefully destroy the existing radioactive nuclei,,
but in nuclear reactions, on the contrary, new radioactive nuclei
could be formed from originally inactive nuclei... In §1.3
"Nuclear reactions" the so-called transmutation technologies
disposal of radioactive waste by neutron irradiation (ADTT) are
discussed, which may be relevant for long-lived and highly active
waste from nuclear reactors.
The activity and half-life of
the relevant radionuclides, as well as the chemical form,
are decisive for the handling and disposal of
radioactive waste. Depending on the half-life and radiotoxicity,
a so-called release level is set for each
radionuclide (or category of radionuclides): it is the value of
activity (total or specific weight or
volume activity) below which the risk of
contamination is negligible and the substance can be released
into the environment without special radiohygienic measures. The
value of the release level is determined on the basis of the
limitation of the possible radiation exposure during internal
contamination by a given radionuclide, using the conversion
factors of ingestion and inhalation
(analyzed above in section "Determination of radiation dose from
internal contamination. MIRD method."). ........
add categories of radionuclides ......
Depending on the state,
radioactive waste can be solid, liquid or gaseous
:
- Solid radioactive
wastes
are collected in properly labeled containers or plastic bags, sorted by activity
and half-life in a special extinction
room,
where it is stored until its activity by natural
decomposition falls below a specified level of release.
At appropriate intervals, they are measured and then
disposed of as non-radioactive waste, eg by incineration
*). Long-term
radioactive waste is "disposed of" by
transporting it to a central repository,
where it is stored in hermetic long-term resistant
containers.
*) During this incineration, it is
a question the disposal of possible hazardous chemicals.
Again, we remind you that the radioactivity
itself cannot be destroyed by incineration !
- Liquid radioactive
wastes
are kept (separately
from the inactive sewerage) to special collection
retention tanks - "decay
tanks",
from where they are discharged into the sewer or
treatment plant only after sufficient decay (approximately 10 half-lives) until their volume activity falls below the
release level. They can then be discharged into a public
sewer or watercourse; there they are further spontaneously mixed and volume diluted with water of the municipal sewage. It is necessary to comply the limit value for the volume
activity of wastewater from the institutions (e.g. for 131I used for thyroid therapy according to the
current standard is 450 Bq/liter), derived from the release level
of the radionuclide. It is suitable of slow continuous or fractionated
discharge of wastewater from the decay sumps, so that the resulting
specific (volume) activity of the discharged waste is as
much as possible significantly lower than the release
level. Some larger workplaces with open radionuclides
have special automated systems for monitoring, diluting
and discharging of wastewater.
For
radioactive waste discharges, it is necessary to
continuously monitoring the activity of wastewater - either by means of a radiometric
detection probe installed in the outlet, or by regular
sampling and their measurement, most sensitively in a
well scintillation detector.
Liquid wastes of long-term radionuclides must be concentrated (volume minimization, or conversion to a solid
state) and
then stored in well-closed containers in a central
repository for a long time.
From the point of view of radiation
protection, it is therefore necessary to
keep
radioactive waste under control until its radioactivity drops to
a sufficiently low level due to spontaneous decay, so that no
threat to the biosphere can occur. This is a difficult problem
for highly active wastes containing radionuclides
with long half- lives; this category mainly includes spent nuclear fuel from fission nuclear reactors (§1.3, section "Nuclear
waste").
Another possibility
is the controlled slow long - term release of radioactive
substances into the natural environment (eg into the
ocean), when two conditions are met :
1. The
current release level of radionuclides must not be exceeded in
order to avoid the possibility of increased radiation exposure
locally at the point of discharge.
2. The total amount of radioactive substances
released must not exceed the "volume capacity" of the
volume of distribution of the given substances in nature, so as
not to increase the natural background radiation in the future.
5.7.
Radiation exposure during radiation diagnostics and therapy
Radiation exposure from diagnostic medical examinations is
generally low and is
almost always justified by the benefit of
accurate diagnosis of possible health disorders and pathological
conditions. These exposures are usually comparable to the doses
we receive continuously from the natural radiation background all
around us. There is no direct evidence that these low radiation
doses could be harmful *) - despite a hypothetical linear
threshold-free dependence and a number of more or less misleading
tables in the radiation protection literature (such as comparisons of radiation and smoking). In contrast, the benefits of a
medical examination are unquestionable and can be very significant
in terms of health !
*) The issues of the radiobiological effect
of low doses of radiation are discussed above in §5.2, section
"Relationship between dose and
biological effect",
section "Problems
of very low doses - are they harmful or beneficial?".
The therapeutic
use of radiation naturally leads to higher exposures,
at which it is necessary to consider and optimize the risks of
treatment against the possible benefits. However, even here, with
proper planning, optimization and strategy, the health
benefits tend to be unquestionable.
Despite the predominant health benefits of
ionizing radiation applications in medical diagnostics and
therapy, issues of radiation protection must be given due
attention here as well.
Radiation
doses and patient protection during diagnostic and therapeutic
procedures
Medical diagnostics and therapy are among the most important
applications of ionizing radiation; it also contributes the most
to the radiation exposure of the population from all artificially
created radiation sources.
The methodology of radiation protection in
medical applications of ionizing radiation - in X-ray
diagnostics, radiotherapy and nuclear medicine - is generally
based on the basic principles of radiation protection mentioned
in §5.3 "Objectives and methods of radiation protection", but has its significant specifics. In
particular, there are do not introduce binding exposure
limits, so as not to limit certain diagnostic and
therapeutic procedures necessary to ensure the health or life of
patients. The slogan is used with a bit of exaggeration: "Each
patient will receive a radiation dose deserves":
the dose needed for accurate diagnosis or effective treatment.
Instead of limits, certain recommended dose values,
so-called guide values - reference levels,
are set as a guideline when performing specific diagnostic or
therapeutic methods (see below "Principle
of optimization").
The principle of
justification of medical exposure
Radiation protection of patients comes out from basic the ethical requirement, that the risk of radiation damage during
diagnostic or therapeutic procedures were balanced (or better, if
possible, prevailed - overcome) by the expected health benefit for the patient. This basic requirement in the medical
application of ionizing radiation is called the principle
of justification of medical exposure in radiation
protection.
Optimization
principle
Another important aspect that contributes in practice to the
balance of radiation risk and benefit, is the analysis of radiation
protection optimization. In X-ray diagnostics, we will use the
lowest exposure that will ensure quality
and well-evaluable images, not a higher exposure. When diagnosing in
nuclear medicine, it is necessary to apply only
such a necessary amount - activity - of radioactive substances (required qualities and purity), which guarantees sufficient
diagnostic information in the images with the lowest
possible radiation exposure of the
patient.
To optimize the amount of applied
radioactivity of various radiopharmaceuticals for individual
examination methods, tables of guideline values, also
called diagnostic reference levels,
which also allow recalculation of applied activity for individual
patients, mostly according to patient weight (even non-standard -
eg children, overweight etc.).
Radiation exposure of patients from X-ray
examinations
By far the most common exposure to ionizing radiation (from artificial sources) in
humans is X-ray diagnostics. In earlier times (around the 60s-70s of the 20th century), when skiagraphy was performed on X-ray films and
sciascopy through fluorescent screens (amplifying
foils and image intensifiers were not yet widespread, digital
flat-panels did not yet exist), the doses
from X-rays were often many tens of mSv. After all, at that time
they were not even evaluated ...
With the development of amplifying foils,
image amplifiers and especially digital display electronic
flat-panels (see §3.2, passage "Electronic
X-ray imaging") radiation doses from X-ray examinations decreased
significantly, in simple images they represent only tenths to
units of mSv. Relatively higher doses (up to tens of mSv) arise
in CT examinations of larger areas (whole chest, abdomen, whole
body), which is, however, balanced by the greater complexity of
diagnostic information. High doses also arise during complicated
interventional X-ray-guided procedures (here,
in addition, the doses are highly variable, depending on the
complexity of the procedure and how easy or difficult it is for
the given patient to "perform" the procedure).
Absorbed radiation dose D
[mGy] in the X-ray examination of a certain area is basically
given by the product of the intensity of X-rays (this is given by
the X-ray current [mA]), the exposure time [s] and the
corresponding coefficients :
D
= G. mA s .
The product of the current I and the exposure time t
indicates the electric amount Q - the total charge
of the electrons - which flowed through the X-ray tube
during the exposure: Q = I. t [mA.s] -
[miliCoulomb] . The electric amount Q
determines the total number of X-rays photons emitted by the
X-ray tube, and thus the signal strength during X-ray imaging.
Coefficient G it includes a number
of factors, such as the efficiency of X-rays production by X-ray
tube, its energy given by the voltage [kV] for X-ray tube,
filtration, distance, tissue absorption coefficients. It is
measured using phantoms, most often water-filled
"aquariums" (for planar X-rays), or cylinders with a
diameter of 16 cm (head) or 32 cm (chest) for CT, equipped with
ionization chambers, thermoluminescence or semiconductor
detectors. The probability of biological stochastic effects is
proportional to this absorbed radiation dose
[mGy] and the size (volume) of the irradiated
area [cm3].
This size - volume - is approximately proportional to the
irradiated area [cm2] in the planar image, or the length [cm] of the CT
scanned area.
In the planar X-ray
diagnostics, this is quantified using the quantity area
dose DAP (Dose Area Product)
[Gy.cm2],
which is the product of the input dose of X-rays and the
size - area S - of the irradiated field: DAP = D. S. The effective
dose Def [mSv] for a patient, expressing the stochastic effects
of radiation on the organism as a whole, is then calculated as
the product :
D
ef
= E DAP . DAP ,
where the coefficient EDAP (regionally normalized effective dose) [mSv Gy-1 cm-2] includes the
averaged tissue (organ) weighting factors wT for structures in the
irradiated area (§5.1 "Basic
quantities of dosimetry", passage "Effective
dose"). Specific
(actual) DAP values during X-ray examinations are measured using
thin plane-parallel ionization chambers mounted on the output
collimator of the X-ray tube - so-called DAP meters
or KAP meters (§3.2,
section "Radiation load during X-ray
examinations").
Typical exposures for planar X-ray imaging
in AP projection are approximately: head 25mAs, DAP = 1Gy.cm2; chest 30mAs, DAP =
0.6Gy.cm2;
abdomen 60mAs, DAP = 1,8Gy.cm2; pelvis 60mAs, DAP = 2Gy.cm2.
During the CT
scan, the X-ray tube circle rotates around the imaging area and
irradiates it on all sides with a substantially uniform dose
within each section. The examined area can be approximated in
terms of dose by a cylindrical shape of
a certain diameter and length. For CT, therefore, dose D
is measured by an ionization chamber inside an acrylate
cylindrical homogeneous phantom with a diameter of 16 cm for the
head and 32 cm for the body and is quantified using the dose
rate index CTDI (CT Dose Index) [mGy], with event.
correction for the pitch factor for spiral instruments.
This dose value determined at the center of the cut is considered
to be an objective indicator (index) doses in tissue.
Here, the probability of biological stochastic effects is again
proportional to the absorbed radiation dose
[mGy] and the size (volume) of the irradiated
area [cm3],
proportional to the scan length [cm]. In X-ray
CT diagnostics, this is expressed using the resulting
linear dose DLP (Dose Length Product) [mGy.cm],
which is the product of the absorbed dose D and
the length L of irradiated area: DLP = D . L (= CTDI . L). The
effective dose Def [mSv] for a patient, expressing the stochastic effects
of radiation on the organism as a whole, is then calculated as
the product of :
D
ef
= E DLP . DLP ,
where the coefficient EDLP (length normalized effective dose) [mSv mGy-1 cm-1] includes averaged tissue
(organ) weighting factors wT for structures in the irradiated area (§5.1 "Basic quantities of dosimetry",
passage "Effective dose").
Typical dose
parameters for CT imaging at 120kV and exposure to 200-400mAs are
approximately: head CTDI = 45mGy, DLP = 640mGy.cm; chest CTDI =
15mGy, DLP = 400mGy.cm; abdomen, pelvis CTDI = 17mGy, DLP =
700mGy.cm.
Using special body
modeling anthropomorphic phantoms, empirical data and
computer simulations, the following approximate values of EDAP and EDLP coefficients were
determined for the basic examined areas of the human body :
| Investigated
area : |
head |
neck |
thorax |
belly |
pelvis |
| EDAP
[mSv Gy-1 cm-2] : |
0.04 |
0.07 |
0.15 |
0.18 |
0.20 |
| EDLP
[mSv mGy-1 cm-1] : |
0.0023 |
0.0054 |
0.017 |
0.017 |
0.019 |
| Tab.5.7.1. Approximate values of the EDAP and EDLP coefficients for the basic imaging areas. |
Note: The radiation
dose in planar X-ray imaging (as opposed to CT) for individual
examined areas is significantly dependent on the geometric
projection used. In our illustrative table, we have given
approximate values of EDAP for antero-posterior AP projection.
Current
computer-controlled X-ray devices (especially CT) determine the
DAP, CTDI and DLP values when imaging a particular patient and
record them in the result protocol (in
DICOM format). The effective dose Def [mSv] can then be
easily determined by multiplying the value of DAP or DLP by the
appropriate EDAP or EDLP coefficient; in the case of a multi-area examination,
the total dose of Def is given by the sum over all parts of the patient's
body examined. This simple method of determining the effective
dose from an X-ray examination is quite sufficient
for the purposes of radiation hygiene - even more complex methods
do not provide more valid results. After all, as stated in §5.1,
the very concept of effective dose is only an
averaged, rough and simplified "qualified estimate"
of complex and individually dependent processes of the biological
effects of radiation...
From the point of view of radiation
protection optimization, guideline values - diagnostic
reference levels - of recommended exposures for planar
skiagraphic images and CT imaging were determined for X-ray
examinations :
| Planar
skiagraphic images |
CT scan |
| Investigated
area |
Input
dose
[mGy] |
DAP
[mGy.cm2] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Abdomen
AP |
5.2 |
2900 |
| Pan
AP |
4.5 |
2000 |
|
| Investigated
area |
CTDI
[mGy] |
DLP
[mGy.cm] |
| Head |
65 |
1100 |
| Neck |
21 |
500 |
| Thorax |
15 |
500 |
| Spine |
32 |
550 |
| Abdomen |
19 |
750 |
| Pan |
25 |
860 |
|
| Tab.5.7.2. Diagnostic reference levels of recommended exposure for planar
skiagraphic images and CT imaging |
Radiation
exposure of patients from radionuclide examinations
is basically determined by applied activity
[MBq] radiotracer (direct proportion), half-life
of the radionuclide used, type and energy of
radiation emitted, and pharmacokinetics of
radiotracer - degree of accumulation of the
radiopharmaceutical in various tissues and organs, as well as its
rate of biological excretion or the residence
time of the radiopharmaceutical in the tissue. These
dependencies are quite complex and individual, in full complexity
the MIRD method
discussed above tries to solve them, Fig.5.5.1.
Based on this method, as well as empirical
determinations and estimates, tables of recalculating
coeficients - conversion factors h
[mSv/MBq] for individual radiopharmaceuticals were compiled,
which allow by simply multiplying the applied activity A
[MBq] to approximately determine the effective dose Def [mSv] for patient: Def = h. A. The values
of conversion factors h (effective
doses/1MBq) and guideline values - diagnostic
reference levels - recommended applied activities (normalized to the patient's weight of 70 kg) for some more frequently used radiopharmaceuticals in
scintigraphy are given in the following table :
| Radionuclide |
Chemical
form |
Effective
dose
h [mSv/MBq] |
Type of
examination |
Applied
activity [MBq] |
| 99m Tc |
| pertechnate TcO 4 |
| phosphates |
| DMSA |
| MAG 3 |
| HIDA |
| macroaggregates |
| MIBI, tetrofosmin |
| HMPAO |
| leukocytes |
| antibodies |
|
| 1.2 . 10 -2 |
| 5.8 . 10 -3 |
| 8.7 . 10 -3 |
| 7.3 . 10 -3 |
| 1.5 . 10 -2 |
| 1.1 . 10 -2 |
| 6.9 . 10 -3 |
| |
| |
| 9.8 . 10 -3 |
|
| thyroid |
| skeleton |
| kidneys static |
| kidneys dynamic |
| liver dynamic |
| perfusion of the lungs |
| myocardial perfusion |
| brain perfusion |
| inflammation |
| tumors |
|
| 200 |
| 800 |
| 150 |
| 250 |
| 250 |
| 200 |
| 800 |
| 800 |
| 600 |
| 800 |
|
| 131 I |
iodide |
24 |
thyroid |
15 |
| 123 I |
| iodide |
| ioflupan, IBZM |
| MIBG |
| |
|
|
| thyroid |
| brain receptors |
| tumors |
| |
|
|
| 111 In |
| leukocytes |
| antibodies |
| DTPA |
| octreotide |
|
| 3.9 . 10 -1 |
| 2.2 . 10 -1 |
| |
| 5.4 . 10 -2 |
|
| inflammation |
| tumors |
| cisternography |
| neuroedocrine
tumors . |
|
|
| 67 Ga |
citrate |
|
inflammation, tumors |
300 |
| 18 F |
FDG, FLT, FMISO, ... |
2.5 . 10 -2 |
tumors |
750 |
| Tab.5.7.3. Diagnostic reference levels of recommended applied activity for the
most frequent examination of scintigraphic diagnostics
and dose conversion factors h for some used
radiopharmaceuticals. |
Note: There is a
significant difference between X-ray diagnostics and
nuclear medicine in the laws of radiation exposure. During
X-ray examination, the source of ionizing radiation is a device
and the radiation dose depends, among other things, on the number
of images performed or on the extent of the area scanned during
CT. In scintigraphy, the source of radiation is not a diagnostic
device, but the patient himself, resp. its investigating organs.
Thus, we can take any number of scintigraphic images without
changing the radiation exposure of the patient. The radiation
exposure of the patient is determined here
already during the application of the radiopharmaceutical
- its type (radionuclide and chemical form) and mainly the
applied activity [MBq].
A certain way to reduce the
radiation exposure of patient after administration of
the radiopharmaceutical, is to influence their biokinetics -
increased hydration with a recommendation for frequent urination (possible administration of a diuretic) for accelerated elimination of radioactive substances
from the body or the application of suitable preparations,
restrictive binding radiopharmaceutical to a particular organ (eg kalium iodine KI for protection of thyroid glands
when administering 131I or 99mTc- labeled radiopharmaceuticals).
Typical
values of radiation exposure of patients from radiation
diagnostics
The average radiation exposure for the most common methods of
X-ray diagnostics (left) and radioisotope diagnostics in
nuclear medicine (right) is given in the following table
5.7.4 (based on IAEA materials). These are approximate average
values, provided that the guideline values of energy, intensity
and exposure time for X-ray diagnostics and guideline values of
applied radioactivity for nuclear medicine methods are observed (here these methods are mostly radiopharmaceuticals
labeled 99mTc, only the last line of positron emission
tomography PET of tumors corresponds to 18F-FDG) :
| X-ray diagnostics |
Radioisotope diagnostics |
| Type of examination
|
Eff. dose
[mSv] |
| Lung image |
0.05 |
| Spine |
0.4 |
| Abdomen |
13 |
| Urography |
2.1 |
| Mammography |
0.5 |
| Angiography |
3 - 9 |
| CT head |
2 |
| CT body (chest,
abdomen) |
7 - 10 |
|
| Type of examination |
Eff. dose
[mSv] |
| Static scintigraphy of the
kidneys |
1.5 |
| Dynamic scintigraphy of the
kidneys |
2.5 |
| Dynamic cholescintigraphy |
2.3 |
| Skeleton scintigraphy |
3.5 |
| Perfusion scintigraphy of the
lungs |
1.2 |
| Thyroid scintigraphy |
2.2 |
| Myocardial perfusion
scintigraphy |
5 |
| PET - tumor diagnostics |
7 |
|
| Tab.5.7.4. Approximate radiation exposure for the
most common methods of X-ray and radioisotope diagnostics |
Standard typical values
of effective radiation doses in X-ray and radioisotope
diagnostics are arranged according to size in the bar graph in
Fig.5.7.1 below. The very lowest radiation doses (almost negligible) are in dental
X-rays imaging (0.008 mSv for
intraoral X-rays and 0.015 mSv for OPG). Similarly, very
low doses
are in bone orthopedic images of
the limbs
and X-rays of the lungs (approximately 0.05-0.07 mSv) . These low effective doses are caused by
two circumstances :
1. To obtain sufficiently high-quality
images, a relatively low exposure (approximately
20mAs) is sufficient,
leading to an absorbed dose of fractions of mGy ;
2. There
are no highly radiosensitive tissues and organs in the scanned
areas (with a high tissue factor wT), but only relatively radioresistant
tissues with wT <0.1 .
Significantly higher radiation doses are in CT examinations, where the X-ray
tube rotates around
the examined area and continuously illuminates it; however,
the higher radiation dose is balanced
here by a
much more detailed diagnostic image. The radiation dose depends
on the size of the scanned area (for CT
head approx. 2mSv, CT of the abdomen up to 10mSv). And the very highest doses, up to tens of mSv, arise in complicated
cases of interventional X-ray- guided
procedures (the last column on the
left in the diagram - here the doses are also considerably
variable, depending on the complexity of the procedure and how
easily or difficultly it is "successful" for the given
patient to perform this procedure...).
All these values of diagnostic radiation
doses fall into the area of very low doses
according to Fig.5.2.3 on the left, where stochastic effects are
not only very small, but also hypothetical (as discussed above in the section "Problems of very low doses - are harmful or
beneficial ?") ..?..
Note to
the table and diagram: Specific values of radiation
doses are in practice highly variable, approx. +- 50%. The table and
diagram are averaged rounded values, more or less indicative (therefore the numerical values of effective doses may
not exactly match), depending on the
devices used and the setting of their parameters, or the values
of the applied activity of radiopharmaceuticals. Some workplaces
emphasize lower exposures and "save"
radiopharmaceuticals (adjust the quality of
images by secondary filtration during evaluation), other workplaces use higher exposures and apply more
activity of radiopharmaceuticals (for
better primary images or shorter examination times)... Overall, it can be said that with the technical
development of instrument electronics and computer evaluation
procedures, radiation doses are gradually decreasing.

Fig.5.7.1 Diagram of approximate typical values of radiation
doses of patients in the most common X-ray and scintigraphic
diagnostic examinations. The blue bars represent X-ray
diagnostics, the red radionuclide scintigraphic diagnostics in
nuclear medicine.
In radiation therapy,
the doses in the target tissues are, of course, quite high
- tens of Gy, cancero-lethal tumor dose,
but also in the surrounding healthy tissues can reach units of Gy
- close tolerance dose. However, even here, with proper
planning, optimization and strategy, the health benefit
is unquestionable (it is discussed
in more detail in §3.6 "Radiotherapy",
section "Physical and radiobiological factors of
radiotherapy").
In therapeutic
applications of radionuclides, the optimally determined amount of
radioactivity is applied - it is determined either according to
verified empirical formulas, or as a lump sum according to the
given diagnosis and the required therapeutic effect (for more details §3.6
"Radiotherapy", passage "Radioisotope therapy"). The activity of each radioactive substance
administered to a patient (especially
therapeutic applications) must be measured on a properly
metrologically calibrated activity meter. The value of the
applied activity is recorded in the
documentation on diagnosis
or therapy; it is an important data for assessing the
effect of therapy and radiation exposure.
......................
Radiation risk of
stochastic effects - distribution of radiopharmaceuticals
according to radiation risk. Assessment of the acceptability of
radiation risk in the context of other occupational and
environmental risks.
Application of radiopharmaceuticals to children - choice
of activity, radiation exposure in children compared to adults.
Application of radiopharmaceuticals to women of
childbearing potential and pregnancy . ....fill in?
In pregnant women, radiation-related
radiodiagnostic procedures should be performed only when
absolutely necessary, choosing the most gentle methods possible with regard to fetal protection.
.......... fill in?
5.8.
Organizational provision of radiation protection
Everyone who uses sources of ionizing radiation is obliged,
within the limits of his competence, to take all necessary
measures to protect the health of himself, his
co-workers and other persons.
The general legislative framework for
working with ionizing radiation is currently the so-called "Atomic
Act" on the peaceful use of nuclear energy and
ionizing radiation and related standards and regulations.
Each country has developed its own "Atomic
Act ", whereas the basic starting points and
procedures are in principle the same - they are based on
knowledge of nuclear and radiation physics, radiobiology,
medicine and related technical fields.
These "Atomic Acts" sets out the
most general rules for working with sources of ionizing
radiation. Especially important are the objectives of radiation
protection - exclusion of deterministic effects and reduction of
stochastic effects to a reasonable minimum, principles of working
with IR -optimization of radiation activities (risk versus
profit), limitation, natural resources, medical exposure .....
Gradual improvement and innovation of
radiation protection regulations are carried out mainly on the
basis of expert recommendations of the International
Commission on Radiological Protection (ICRP).
Central institutes and offices for nuclear
safety and radiation protection are established to
supervise and coordinate the whole set of measures for the safe
use of ionizing radiation sources. In addition to legislative
activities, these instituions assesses projects
of workplaces with sources of ionizing radiation, issues relevant
permits and performs inspection activities at these
workplaces.
In addition, a supervisory worker of
radiation protection is established at each workplace with
ionizing radiation, which deals with radiation protection issues
on site and keeps the relevant documentation. At the larger
workplaces of nuclear medicine, a technical and physical
department has been established, which, together with other
physical and technical issues of the workplace, also provides a
radiation protection methodology from a professional point of
view. In some large medical institutes, a central Department
of Medical Radiation Physics and Hygiene has been
established, which coordinates all issues of radiation
application and radiation protection.
A set of main principles, measures and methodology of
measuring procedures to ensure the optimal level of radiation
protection at a particular workplace are written in the so-called
Monitoring Program of the workplace (what is measured, how
often is measured, where is measured, how and what is measured,
interpretation of measurement results and their documentation).
Part of the monitoring program is also the determination of reference
levels - recording, investigation, intervention.
Another related material is the Quality Assurance Program
for diagnostic and therapeutic activities of the workplace, which
is a set of control and adjustment activities to ensure the
proper functioning of devices and the required quality of
radiopharmaceuticals; this is a condition for accurate and
reliable measurement and examination results. This is related to
radiation protection through optimization between the benefits
and risks of ionizing radiation application: the
more valid the diagnostic results and the better the effects of
therapy, the more the health benefits of patients outweigh the
risk of ionizing radiation side effects - and vice versa.
The set of measures, including decontamination procedures and
control measurements in the event of radiation accidents and
other extraordinary events at the workplace, are summarized in
the workplace Emergency code. Also the Operating rules
of the workplace contain a number of specific principles for
correct and safe work with sources of ionizing radiation.
As organizational and legislative issues are far removed
from the professional focus of the author (physics), it will be
appropriate to refer to the details of, for example,
www-materials: https://www.icrp.org.......(Some aspects are briefly mentioned also in the syllabus
" Radiation
protection ").
Author's
personal note: Unnecessary
bureaucracy in radiation protection
As mentioned above, currently the field of applications
of ionizing radiation in terms of radiation protection
is monitored, coordinated and secured as thoroughly
as perhaps no other field of human activity. In general, this
must certainly be acknowledged very positively.
However, as a physicist working for more than 40 years in the
field of nuclear and radiation physics, I would like to make a
small critical remarks :
It seems to me that this overall very
high-quality concept of radiation protection is sometimes
implemented and interpreted in recent years, perhaps
overly bureaucratically... It is sometimes based on
insignificant details, requirements important in the areas of
high activities and radiation intensities are mechanically
transferred to small workplaces (such
as nuclear medicine), where there are virtually
no radiation risks. The number of official
"papers" and documents and their scope have grown
enormously. They emphasize and broadly describe the tasks,
powers, signatures of irrelevant persons -
various those statutory representatives, directors, deputies and
other officials who have nothing to do with radiation activity
and often do not even know about its nature and existence ...
Experts in radiation protection are
certainly not responsible for this situation (perhaps with the exception of isolated cases of servile
efforts to be "more papal than the pope" and busily
multiply irrelevant regulations...). This
is related to the more general trend towards a hierarchical
organization of Western society, the growth of
bureaucracy and the replacement of real law by formal
justice - the golden rule "the letter kills, the
spirit revives" is forgotten.
In the field of radiation applications, as
well as radiation protection, almost exclusively professionally
well-founded people work, well acquainted with the
principles of working with radioactivity and ionizing radiation.
These workers eruditely carry out their
professional work and implement radiation protection "as
is should be", to the best of their knowledge and
conscience. They should be given more confidence, without
unnecessary "buzzings" and enormous administrative
burden ..!..
Incidentally, a similar bureaucratic
approach are seen in regulations and requirements for
the preparation and filling of radiopharmaceuticals
for nuclear medicine (§4.8, part
"Radionuclides and radiopharmaceuticals", note "Radiopharmaceuticals - bureaucracy").
Vojtech Ullmann


















